Differential Effects of MicroRNAs on Glioblastoma Growth and Migration
- PMID: 24705102
- PMCID: PMC3899955
- DOI: 10.3390/genes4010046
Differential Effects of MicroRNAs on Glioblastoma Growth and Migration
Abstract
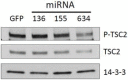
Glioblastoma multiforme is characterized by rapid proliferation, aggressive metastatic potential, and resistance to radio- and chemotherapy. The matricellular protein CYR61 regulates cellular proliferation and migration and is highly expressed in Glioblastomas. MicroRNAs are 22-nucleotides long RNAs that regulate gene expression post-transcriptionally. Here, we utilized the LN229 glioblastoma cell line and found that CYR61 is a target of miR-136, miR-155, and miR-634. Over-expression of miR-136 and miR-634 miRNAs negatively affected proliferation, but not migration, while expression of miR-155 reduced migration but did not affect the proliferation of LN229 cells. Investigation of the molecular mechanisms affected by expression of miR-634 revealed an increased phosphorylation of p70S6 kinase, suggesting an induction of the mammalian target of rapamycin (mTOR) complex 1 pathway. Additionally, in miR-634 overexpressing cells, TSC2, a negative regulator of mTOR signaling, was found to be decreased. Altogether, our study provides insights on the differential roles of miRs-136, -155, and -634 in regulating glioblastoma cell growth and migration, and how microRNAs could be manipulated to decrease the aggressiveness and metastatic potential of tumor cells.
Figures







References
-
- Nagasawa D.T., Chow F., Yew A., Kim W., Cremer N., Yang I. Temozolomide and other potential agents for the treatment of glioblastoma multiforme. Neurosurg. Clin. N Am. 2012;23:307–322, ix. - PubMed
-
- Drukala J., Urbanska K., Wilk A., Grabacka M., Wybieralska E., Del Valle L., Madeja Z., Reiss K. ROS accumulation and IGF-IR inhibition contribute to fenofibrate/PPARalpha—mediated inhibition of glioma cell motility in vitro. Mol. Cancer. 2010;9:159. doi: 10.1186/1476-4598-9-159. - DOI - PMC - PubMed
-
- Furnari F.B., Huang H.J., Cavenee W.K. Genetics and malignant progression of human brain tumours. Cancer Surv. 1995;25:233–275. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

