Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial
- PMID: 24705119
- PMCID: PMC4029928
- DOI: 10.1161/CIRCULATIONAHA.113.006191
Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial
Abstract
Background: In the Single Ventricle Reconstruction (SVR) trial, 1-year transplantation-free survival was better for the Norwood procedure with right ventricle-to-pulmonary artery shunt (RVPAS) compared with a modified Blalock-Taussig shunt (MBTS). At 3 years, we compared transplantation-free survival, echocardiographic right ventricular ejection fraction, and unplanned interventions in the treatment groups.
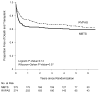
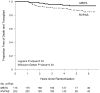
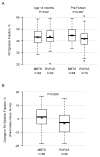
Methods and results: Vital status and medical history were ascertained from annual medical records, death indexes, and phone interviews. The cohort included 549 patients randomized and treated in the SVR trial. Transplantation-free survival for the RVPAS versus MBTS groups did not differ at 3 years (67% versus 61%; P=0.15) or with all available follow-up of 4.8±1.1 years (log-rank P=0.14). Pre-Fontan right ventricular ejection fraction was lower in the RVPAS group than in the MBTS group (41.7±5.1% versus 44.7±6.0%; P=0.007), and right ventricular ejection fraction deteriorated in RVPAS (P=0.004) but not MBTS (P=0.40) subjects (pre-Fontan minus 14-month mean, -3.25±8.24% versus 0.99±8.80%; P=0.009). The RVPAS versus MBTS treatment effect had nonproportional hazards (P=0.004); the hazard ratio favored the RVPAS before 5 months (hazard ratio=0.63; 95% confidence interval, 0.45-0.88) but the MBTS beyond 1 year (hazard ratio=2.22; 95% confidence interval, 1.07-4.62). By 3 years, RVPAS subjects had a higher incidence of catheter interventions (P<0.001) with an increasing HR over time (P=0.005): <5 months, 1.14 (95% confidence interval, 0.81-1.60); from 5 months to 1 year, 1.94 (95% confidence interval, 1.02-3.69); and >1 year, 2.48 (95% confidence interval, 1.28-4.80).
Conclusions: By 3 years, the Norwood procedure with RVPAS compared with MBTS was no longer associated with superior transplantation-free survival. Moreover, RVPAS subjects had slightly worse right ventricular ejection fraction and underwent more catheter interventions with increasing hazard ratio over time.
Clinical trial registration url: http://www.clinicaltrials.gov. Unique identifier: NCT00115934.
Keywords: Norwood procedures; cardiac surgical procedures; heart defects, congenital; heart diseases; heart ventricles.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Figures
Comment in
-
Single ventricle reconstruction trial: a work in progress.Circulation. 2014 May 20;129(20):2000-1. doi: 10.1161/CIRCULATIONAHA.114.009594. Epub 2014 Apr 4. Circulation. 2014. PMID: 24705120 Free PMC article. No abstract available.
References
-
- Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects--United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;562:25–29. - PubMed
-
- Ohye RG, Ludomirsky A, Devaney EJ, Bove EL. Comparison of right ventricle to pulmonary artery conduit and modified Blalock-Taussig shunt hemodynamics after the Norwood operation. Ann Thorac Surg. 2004;783:1090–1093. - PubMed
-
- Pizarro C, Mroczek T, Malec E, Norwood WI. Right ventricle to pulmonary artery conduit reduces interim mortality after stage 1 Norwood for hypoplastic left heart syndrome. Ann Thorac Surg. 2004;78:1959–1963. - PubMed
-
- Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- HL068288/HL/NHLBI NIH HHS/United States
- HL068285/HL/NHLBI NIH HHS/United States
- U01 HL068290/HL/NHLBI NIH HHS/United States
- U10 HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068281/HL/NHLBI NIH HHS/United States
- U10 HL109737/HL/NHLBI NIH HHS/United States
- HL068290/HL/NHLBI NIH HHS/United States
- U01 HL068269/HL/NHLBI NIH HHS/United States
- U10 HL109816/HL/NHLBI NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- U01 HL068279/HL/NHLBI NIH HHS/United States
- HL068279/HL/NHLBI NIH HHS/United States
- U01 HL068288/HL/NHLBI NIH HHS/United States
- U01 HL068270/HL/NHLBI NIH HHS/United States
- HL085057/HL/NHLBI NIH HHS/United States
- HL068281/HL/NHLBI NIH HHS/United States
- U01 HL068292/HL/NHLBI NIH HHS/United States
- HL068269/HL/NHLBI NIH HHS/United States
- HL068270/HL/NHLBI NIH HHS/United States
- U01 HL085057/HL/NHLBI NIH HHS/United States
- U10 HL109778/HL/NHLBI NIH HHS/United States
- U01 HL068285/HL/NHLBI NIH HHS/United States
- HL068292/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

