Synthesis and propagation of complement C3 by microglia/monocytes in the aging retina
- PMID: 24705166
- PMCID: PMC3976274
- DOI: 10.1371/journal.pone.0093343
Synthesis and propagation of complement C3 by microglia/monocytes in the aging retina
Abstract
Introduction: Complement activation is thought to contribute to the pathogenesis of age-related macular degeneration (AMD), which may be mediated in part by para-inflammatory processes. We aimed to investigate the expression and localization of C3, a crucial component of the complement system, in the retina during the course of aging.
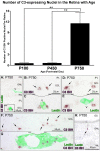
Methods: SD rats were born and reared in low-light conditions, and euthanized at post-natal (P) days 100, 450, or 750. Expression of C3, IBA1, and Ccl- and Cxcl- chemokines was assessed by qPCR, and in situ hybridization. Thickness of the ONL was assessed in retinal sections as a measure of photoreceptor loss, and counts were made of C3-expressing monocytes.
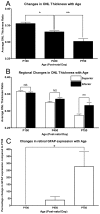
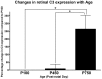
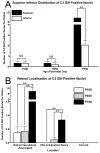
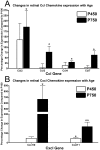
Results: C3 expression increased significantly at P750, and correlated with thinning of the ONL, at P750, and up-regulation of GFAP. In situ hybridization showed that C3 was expressed by microglia/monocytes, mainly from within the retinal vasculature, and occasionally the ONL. The number of C3-expressing microglia increased significantly by P750, and coincided spatiotemporally with thinning of the ONL, and up-regulation of Ccl- and Cxcl- chemokines.
Conclusions: Our data suggest that recruited microglia/monocytes contribute to activation of complement in the aging retina, through local expression of C3 mRNA. C3 expression coincides with age-related thinning of the ONL at P750, although it is unclear whether the C3-expressing monocytes are a cause or consequence. These findings provide evidence of activation of complement during natural aging, and may have relevance to cellular events underling the pathogenesis of age-related retinal diseases.
Conflict of interest statement
Figures


References
-
- Bonnel S, Mohand-Said S, Sahel JA (2003) The aging of the retina. Exp Gerontol 38: 825–831. - PubMed
-
- Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP (2003) Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 48: 257–293. - PubMed
-
- Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, et al. (2009) Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol 127: 533–540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

