Long-term exposure to decabrominated diphenyl ether impairs CD8 T-cell function in adult mice
- PMID: 24705197
- PMCID: PMC4085518
- DOI: 10.1038/cmi.2014.16
Long-term exposure to decabrominated diphenyl ether impairs CD8 T-cell function in adult mice
Abstract
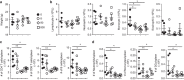
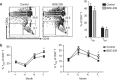
Polybrominated diphenyl ethers (PBDEs) are ubiquitous environmental pollutants that accumulate to high levels in human populations that are subject to occupational or regional industry exposure. PBDEs have been shown to affect human neuronal, endocrine and reproductive systems, but their effect on the immune system is not well understood. In this study, experimental adult mice were intragastrically administered 2,2',3,3',4,4',5,5',6,6'-decabromodiphenyl ether (BDE-209) at doses of 8, 80 or 800 mg/kg of body weight (bw) at 2-day intervals. Our results showed that continuous exposure to BDE-209 resulted in high levels of BDE-209 in the plasma that approached the levels found in people who work in professions with high risks of PDBE exposure. Reduced leukocytes, decreased cytokine (IFN-γ, IL-2 and TNF-α) production and lower CD8 T-cell proliferation were observed in the mice exposed to BDE-209. Additionally, mice with long-term BDE-209 exposure had lower numbers of antigen-specific CD8 T cells after immunization with recombinant Listeria monocytogenes expressing ovalbumin (rLm-OVA) and the OVA-specific CD8 T cells had reduced functionality. Taken together, our study demonstrates that continuous BDE-209 exposure causes adverse effects on the number and functionality of immune cells in adult mice.
Figures




Similar articles
-
Long-term exposure to high levels of decabrominated diphenyl ether inhibits CD4 T-cell functions in C57Bl/6 mice.J Appl Toxicol. 2016 Sep;36(9):1112-9. doi: 10.1002/jat.3270. Epub 2015 Dec 18. J Appl Toxicol. 2016. PMID: 26682527
-
Simulating long-term occupational exposure to decabrominated diphenyl ether using C57BL/6 mice: biodistribution and pathology.Chemosphere. 2015 Jun;128:118-24. doi: 10.1016/j.chemosphere.2015.01.012. Epub 2015 Feb 14. Chemosphere. 2015. PMID: 25687576
-
Age-associated alterations in CD8α+ dendritic cells impair CD8 T-cell expansion in response to an intracellular bacterium.Aging Cell. 2012 Dec;11(6):968-77. doi: 10.1111/j.1474-9726.2012.00867.x. Epub 2012 Aug 30. Aging Cell. 2012. PMID: 22862959 Free PMC article.
-
[Research progress of health effect of polybrominated diphenyl ethers].Zhonghua Yu Fang Yi Xue Za Zhi. 2016 Jun;50(6):559-62. doi: 10.3760/cma.j.issn.0253-9624.2016.06.018. Zhonghua Yu Fang Yi Xue Za Zhi. 2016. PMID: 27256741 Review. Chinese.
-
Neurodevelopmental effects of decabromodiphenyl ether (BDE-209) and implications for the reference dose.Regul Toxicol Pharmacol. 2009 Jun;54(1):91-104. doi: 10.1016/j.yrtph.2009.02.006. Epub 2009 Feb 26. Regul Toxicol Pharmacol. 2009. PMID: 19249332 Review.
Cited by
-
Low-dose exposure to PBDE disrupts genomic integrity and innate immunity in mammary tissue.Front Genet. 2022 Aug 12;13:904607. doi: 10.3389/fgene.2022.904607. eCollection 2022. Front Genet. 2022. PMID: 36035174 Free PMC article.
-
Deep Surveying of the Transcriptional and Alternative Splicing Signatures for Decidual CD8+ T Cells at the First Trimester of Human Healthy Pregnancy.Front Immunol. 2018 May 4;9:937. doi: 10.3389/fimmu.2018.00937. eCollection 2018. Front Immunol. 2018. PMID: 29780389 Free PMC article.
-
Characterization of T follicular helper cells in allogeneic normal pregnancy and PDL1 blockage-induced abortion.Sci Rep. 2016 Nov 7;6:36560. doi: 10.1038/srep36560. Sci Rep. 2016. PMID: 27819282 Free PMC article.
-
BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway.Molecules. 2023 Feb 21;28(5):2036. doi: 10.3390/molecules28052036. Molecules. 2023. PMID: 36903282 Free PMC article.
-
Peripheral CD19hi B cells exhibit activated phenotype and functionality in promoting IgG and IgM production in human autoimmune diseases.Sci Rep. 2017 Oct 24;7(1):13921. doi: 10.1038/s41598-017-14089-2. Sci Rep. 2017. PMID: 29066741 Free PMC article.
References
-
- Rahman F, Langford KH, Scrimshaw MD, Lester JN. Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ. 2001;275:1–17. - PubMed
-
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs—a review of levels and sources. Int J Hyg Environ Health. 2009;212:109–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

