Parathyroid hormone administration improves bone marrow microenvironment and partially rescues haematopoietic defects in Bmi1-null mice
- PMID: 24705625
- PMCID: PMC3976339
- DOI: 10.1371/journal.pone.0093864
Parathyroid hormone administration improves bone marrow microenvironment and partially rescues haematopoietic defects in Bmi1-null mice
Abstract
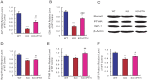
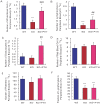
The epigenetic regulator Bmi1 is key in haematopoietic stem cells, and its inactivation leads to defects in haematopoiesis. Parathyroid hormone (PTH), an important modulator of bone homeostasis, also regulates haematopoiesis, so we asked whether PTH administration improves bone marrow microenvironment and rescues the haematopoietic defects in Bmi1-null mice. The mice were treated with PTH1-34 (containing the first 34 residues of mature PTH), an anabolic drug currently used for treating osteoporosis, and compared with the vehicle-treated Bmi1-/- and wild-type littermates in terms of skeletal and haematopoietic phenotypes. We found that the administration significantly increased all parameters related to osteoblastic bone formation and significantly reduced the adipocyte number and PPARγ expression. The bone marrow cellularity, numbers of haematopoietic progenitors and stem cells in the femur, and numbers of lymphocytes and other white blood cells in the peripheral blood all increased significantly when compared to vehicle-treated Bmi1-/- mice. Moreover, the number of Jagged1-positive cells and percentage of Notch intracellular domain-positive bone marrow cells and protein expression levels of Jagged1 and NICD in bone tissue were also increased in Bmi1-/- mice upon PTH1-34 administration,whereas the up-regulation of PTH on both Notch1 and Jagged1 gene expression was blocked by the Notch inhibitor DAPT administration. These results thus indicate that PTH administration activates the notch pathway and partially rescues haematopoietic defects in Bmi1-null mice, further suggesting that haematopoietic defects in the animals are not only a result of reduced self-renewal of haematopoietic stem cells but also due to impaired bone marrow microenvironment.
Conflict of interest statement
Figures




References
-
- Trumpp A, Essers M, Wilson A (2010) Awakening dormant haematopoietic stem cells. Nat Rev Immunol 10: 201–209. - PubMed
-
- Jones DL, Wagers AJ (2008) No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 9: 11–21. - PubMed
-
- Frenette PS, Pinho S, Lucas D, Scheiermann C (2013) Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol 31: 285–316. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, et al. (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

