The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury
- PMID: 24705722
- PMCID: PMC4025060
- DOI: 10.1152/ajplung.00135.2013
The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury
Abstract
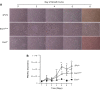
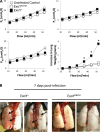
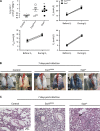
Exoenzyme Y (ExoY) is a Pseudomonas aeruginosa toxin that is introduced into host cells through the type 3 secretion system (T3SS). Once inside the host cell cytoplasm, ExoY generates cyclic nucleotides that cause tau phosphorylation and microtubule breakdown. Microtubule breakdown causes interendothelial cell gap formation and tissue edema. Although ExoY transiently induces interendothelial cell gap formation, it remains unclear whether ExoY prevents repair of the endothelial cell barrier. Here, we test the hypothesis that ExoY intoxication impairs recovery of the endothelial cell barrier following gap formation, decreasing migration, proliferation, and lung repair. Pulmonary microvascular endothelial cells (PMVECs) were infected with P. aeruginosa strains for 6 h, including one possessing an active ExoY (PA103 exoUexoT::Tc pUCPexoY; ExoY(+)), one with an inactive ExoY (PA103ΔexoUexoT::Tc pUCPexoY(K81M); ExoY(K81M)), and one that lacks PcrV required for a functional T3SS (ΔPcrV). ExoY(+) induced interendothelial cell gaps, whereas ExoY(K81M) and ΔPcrV did not promote gap formation. Following gap formation, bacteria were removed and endothelial cell repair was examined. PMVECs were unable to repair gaps even 3-5 days after infection. Serum-stimulated growth was greatly diminished following ExoY intoxication. Intratracheal inoculation of ExoY(+) and ExoY(K81M) caused severe pneumonia and acute lung injury. However, whereas the pulmonary endothelial cell barrier was functionally improved 1 wk following ExoY(K81M) infection, pulmonary endothelium was unable to restrict the hyperpermeability response to elevated hydrostatic pressure following ExoY(+) infection. In conclusion, ExoY is an edema factor that chronically impairs endothelial cell barrier integrity following lung injury.
Keywords: cyclase; microtubules; permeability; pulmonary edema; tau.
Copyright © 2014 the American Physiological Society.
Figures



Similar articles
-
Pseudomonas aeruginosa ExoY infection of pulmonary microvascular endothelial cells releases cyclic nucleotides into the extracellular compartment.Am J Physiol Lung Cell Mol Physiol. 2024 Nov 1;327(5):L756-L768. doi: 10.1152/ajplung.00038.2024. Epub 2024 Sep 24. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39316682
-
cUMP elicits interendothelial gap formation during Pseudomonas aeruginosa infection.Am J Physiol Lung Cell Mol Physiol. 2024 Sep 1;327(3):L395-L405. doi: 10.1152/ajplung.00164.2023. Epub 2024 Jul 30. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39076085 Free PMC article.
-
Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY.Circ Res. 2004 Jul 23;95(2):196-203. doi: 10.1161/01.RES.0000134922.25721.d9. Epub 2004 Jun 10. Circ Res. 2004. PMID: 15192021
-
The Pseudomonas aeruginosa Exoenzyme Y: A Promiscuous Nucleotidyl Cyclase Edema Factor and Virulence Determinant.Handb Exp Pharmacol. 2017;238:67-85. doi: 10.1007/164_2016_5003. Handb Exp Pharmacol. 2017. PMID: 28181005 Free PMC article. Review.
-
ExoY, an actin-activated nucleotidyl cyclase toxin from P. aeruginosa: A minireview.Toxicon. 2018 Jul;149:65-71. doi: 10.1016/j.toxicon.2017.12.046. Epub 2017 Dec 16. Toxicon. 2018. PMID: 29258848 Review.
Cited by
-
Research Progress on Small Molecular Inhibitors of the Type 3 Secretion System.Molecules. 2022 Nov 30;27(23):8348. doi: 10.3390/molecules27238348. Molecules. 2022. PMID: 36500441 Free PMC article. Review.
-
ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation.Biochem Biophys Res Commun. 2014 Jul 18;450(1):870-4. doi: 10.1016/j.bbrc.2014.06.088. Epub 2014 Jun 24. Biochem Biophys Res Commun. 2014. PMID: 24971548 Free PMC article.
-
Pseudomonas aeruginosa exoenzymes U and Y induce a transmissible endothelial proteinopathy.Am J Physiol Lung Cell Mol Physiol. 2016 Feb 15;310(4):L337-53. doi: 10.1152/ajplung.00103.2015. Epub 2015 Dec 4. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26637633 Free PMC article.
-
Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors.Int J Mol Sci. 2021 Mar 18;22(6):3128. doi: 10.3390/ijms22063128. Int J Mol Sci. 2021. PMID: 33803907 Free PMC article. Review.
-
Pseudomonas aeruginosa Cytotoxins: Mechanisms of Cytotoxicity and Impact on Inflammatory Responses.Cells. 2023 Jan 3;12(1):195. doi: 10.3390/cells12010195. Cells. 2023. PMID: 36611990 Free PMC article. Review.
References
-
- Ahuja N, Kumar P, Bhatnagar R. The adenylate cyclase toxins. Crit Rev Microbiol 30: 187–196, 2004. - PubMed
-
- Baig S, van Helmond Z, Love S. Tau hyperphosphorylation affects Smad 2/3 translocation. Neuroscience 163: 561–570, 2009. - PubMed
-
- Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879–1890, 2004. - PubMed
-
- Braun N, Papadopoulos T, Muller-Hermelink HK. Cell cycle dependent distribution of the proliferation-associated Ki-67 antigen in human embryonic lung cells. Virchows Arch B Cell Pathol Incl Mol Pathol 56: 25–33, 1988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

