Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels
- PMID: 24705725
- PMCID: PMC4042193
- DOI: 10.1152/ajplung.00015.2014
Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels
Abstract
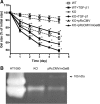
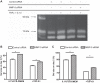
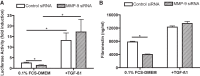
Matrix metalloproteinase-9 (MMP-9) is a matrix-degrading enzyme implicated in many biological processes, including inflammation. It is produced by many cells, including fibroblasts. When cultured in three-dimensional (3D) collagen gels, fibroblasts contract the surrounding matrix, a function that is thought to model the contraction that characterizes both normal wound repair and fibrosis. The current study was designed to evaluate the role of endogenously produced MMP-9 in fibroblast contraction of 3D collagen gels. Fibroblasts from mice lacking expression of MMP-9 and human lung fibroblasts (HFL-1) transfected with MMP-9 small-interfering RNA (siRNA) were used. Fibroblasts were cast into type I collagen gels and floated in culture medium with or without transforming growth factor (TGF)-β1 for 5 days. Gel size was determined daily using an image analysis system. Gels made from MMP-9 siRNA-treated human fibroblasts contracted less than control fibroblasts, as did fibroblasts incubated with a nonspecific MMP inhibitor. Similarly, fibroblasts cultured from MMP-9-deficient mice contracted gels less than did fibroblasts from control mice. Transfection of the MMP-9-deficient murine fibroblasts with a vector expressing murine MMP-9 restored contractile activity to MMP-9-deficient fibroblasts. Inhibition of MMP-9 reduced active TGF-β1 and reduced several TGF-β1-driven responses, including activity of a Smad3 reporter gene and production of fibronectin. Because TGF-β1 also drives fibroblast gel contraction, this suggests the mechanism for MMP-9 regulation of contraction is through the generation of active TGF-β1. This study provides direct evidence that endogenously produced MMP-9 has a role in regulation of tissue contraction of 3D collagen gels mediated by fibroblasts.
Keywords: lung; repair; transforming growth factor-β.
Copyright © 2014 the American Physiological Society.
Figures




References
-
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 28: 12–24, 2003 - PubMed
-
- Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, Kawakami Y. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med 159: 1985–1991, 1999 - PubMed
-
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4: 197–250, 1993 - PubMed
-
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med 342: 1350–1358, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

