LIMLE, a new molecule over-expressed following activation, is involved in the stimulatory properties of dendritic cells
- PMID: 24705920
- PMCID: PMC3976354
- DOI: 10.1371/journal.pone.0093894
LIMLE, a new molecule over-expressed following activation, is involved in the stimulatory properties of dendritic cells
Abstract
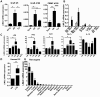
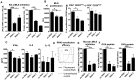
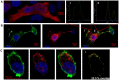
Dendritic cells are sentinels of the immune system distributed throughout the body, that following danger signals will migrate to secondary lymphoid organs to induce effector T cell responses. We have identified, in a rodent model of graft rejection, a new molecule expressed by dendritic cells that we have named LIMLE (RGD1310371). To characterize this new molecule, we analyzed its regulation of expression and its function. We observed that LIMLE mRNAs were rapidly and strongly up regulated in dendritic cells following inflammatory stimulation. We demonstrated that LIMLE inhibition does not alter dendritic cell maturation or cytokine production following Toll-like-receptor stimulation. However, it reduces their ability to stimulate effector T cells in a mixed leukocyte reaction or T cell receptor transgenic system. Interestingly, we observed that LIMLE protein localized with actin at some areas under the plasma membrane. Moreover, LIMLE is highly expressed in testis, trachea, lung and ciliated cells and it has been shown that cilia formation bears similarities to formation of the immunological synapse which is required for the T cell activation by dendritic cells. Taken together, these data suggest a role for LIMLE in specialized structures of the cytoskeleton that are important for dynamic cellular events such as immune synapse formation. In the future, LIMLE may represent a new target to reduce the capacity of dendritic cells to stimulate T cells and to regulate an immune response.
Conflict of interest statement
Figures
Similar articles
-
WASp-dependent actin cytoskeleton stability at the dendritic cell immunological synapse is required for extensive, functional T cell contacts.J Leukoc Biol. 2016 May;99(5):699-710. doi: 10.1189/jlb.2A0215-050RR. Epub 2015 Nov 20. J Leukoc Biol. 2016. PMID: 26590149 Free PMC article.
-
Imaging the immunological synapse between dendritic cells and T cells.J Immunol Methods. 2015 Aug;423:40-4. doi: 10.1016/j.jim.2015.04.029. Epub 2015 May 9. J Immunol Methods. 2015. PMID: 25967948
-
Inhibition of immune synapse by altered dendritic cell actin distribution: a new pathway of mesenchymal stem cell immune regulation.J Immunol. 2010 Nov 1;185(9):5102-10. doi: 10.4049/jimmunol.1001332. Epub 2010 Oct 1. J Immunol. 2010. PMID: 20889545
-
L-plastin regulates the stability of the immune synapse of naive and effector T-cells.Adv Biol Regul. 2017 Jan;63:107-114. doi: 10.1016/j.jbior.2016.09.009. Epub 2016 Sep 27. Adv Biol Regul. 2017. PMID: 27720134 Review.
-
Toll-like receptor signaling and regulation of cytokine gene expression in the immune system.Biotechniques. 2002 Oct;Suppl:66-8, 70, 72 passim. Biotechniques. 2002. PMID: 12395929 Review.
Cited by
-
The Role of Mononuclear Phagocytes in the Testes and Epididymis.Int J Mol Sci. 2022 Dec 20;24(1):53. doi: 10.3390/ijms24010053. Int J Mol Sci. 2022. PMID: 36613494 Free PMC article. Review.
References
-
- Medzhitov R, Janeway C Jr (2000) Innate immunity. N Engl J Med 343: 338–344. - PubMed
-
- Trombetta ES, Mellman I (2005) Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23: 975–1028. - PubMed
-
- van Helden SF, Krooshoop DJ, Broers KC, Raymakers RA, Figdor CG, et al. (2006) A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J Immunol 177: 1567–1574. - PubMed
-
- Watts C, Zaru R, Prescott AR, Wallin RP, West MA (2007) Proximal effects of Toll-like receptor activation in dendritic cells. Curr Opin Immunol 19: 73–78. - PubMed
-
- Nobile C, Lind M, Miro F, Chemin K, Tourret M, et al. (2008) Cognate CD4+ T-cell-dendritic cell interactions induce migration of immature dendritic cells through dissolution of their podosomes. Blood 111: 3579–3590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

