Patient-derived xenografts for individualized care in advanced sarcoma
- PMID: 24705963
- PMCID: PMC4298787
- DOI: 10.1002/cncr.28696
Patient-derived xenografts for individualized care in advanced sarcoma
Erratum in
- Cancer. 2014 Nov 15;120(22):3588. Zacharoulis, Stergios [added]
Abstract
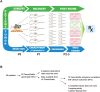
Background: Patients with advanced, metastatic sarcoma have a poor prognosis, and the overall benefit from the few standard-of-care therapeutics available is small. The rarity of this tumor, combined with the wide range of subtypes, leads to difficulties in conducting clinical trials. The authors previously reported the outcome of patients with a variety of common solid tumors who received treatment with drug regimens that were first tested in patient-derived xenografts using a proprietary method ("TumorGrafts").
Methods: Tumors resected from 29 patients with sarcoma were implanted into immunodeficient mice to identify drug targets and drugs for clinical use. The results of drug sensitivity testing in the TumorGrafts were used to personalize cancer treatment.
Results: Of 29 implanted tumors, 22 (76%) successfully engrafted, permitting the identification of treatment regimens for these patients. Although 6 patients died before the completion of TumorGraft testing, a correlation between TumorGraft results and clinical outcome was observed in 13 of 16 (81%) of the remaining individuals. No patients progressed during the TumorGraft-predicted therapy.
Conclusions: The current data support the use of the personalized TumorGraft model as an investigational platform for therapeutic decision-making that can guide treatment for rare tumors such as sarcomas. A randomized phase 3 trial versus physician's choice is warranted.
Keywords: TumorGraft; biomarker; chemotherapy; mice; prediction; response; sarcoma; trial.
© 2014 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Figures





Comment in
-
Patient-derived sarcoma xenografts for individual-patient selection of chemotherapy--ready for prime time?Cancer. 2014 Jul 1;120(13):1917-9. doi: 10.1002/cncr.28694. Epub 2014 Apr 4. Cancer. 2014. PMID: 24706515 No abstract available.
Similar articles
-
Large institutional experience with dose-intensive chemotherapy and stem cell support in the management of sarcoma patients.Oncology. 2007;73(1-2):58-64. doi: 10.1159/000120629. Epub 2008 Mar 11. Oncology. 2007. PMID: 18334832
-
Comparison of the diagnostic performances of core needle biopsy in myxoid versus non-myxoid tumors.Eur J Surg Oncol. 2019 Jul;45(7):1293-1298. doi: 10.1016/j.ejso.2019.05.001. Epub 2019 May 2. Eur J Surg Oncol. 2019. PMID: 31085026
-
Clinical genomic profiling to identify actionable alterations for investigational therapies in patients with diverse sarcomas.Oncotarget. 2017 Jun 13;8(24):39254-39267. doi: 10.18632/oncotarget.16845. Oncotarget. 2017. PMID: 28424409 Free PMC article.
-
[Cutaneous and subcutaneous sarcomas].Ann Chir Plast Esthet. 1998 Aug;43(4):421-38. Ann Chir Plast Esthet. 1998. PMID: 9926474 Review. French.
-
Heterologous and rare homologous sarcomas of the uterine corpus: a clinicopathologic review.Adv Anat Pathol. 2011 Jan;18(1):60-74. doi: 10.1097/PAP.0b013e3182026be7. Adv Anat Pathol. 2011. PMID: 21169739 Review.
Cited by
-
Microvessels Density in Uterine Leiomyosarcoma.Biomed Res Int. 2015;2015:475305. doi: 10.1155/2015/475305. Epub 2015 Jun 16. Biomed Res Int. 2015. PMID: 26161403 Free PMC article.
-
Genome-Informed Targeted Therapy for Osteosarcoma.Cancer Discov. 2019 Jan;9(1):46-63. doi: 10.1158/2159-8290.CD-17-1152. Epub 2018 Sep 28. Cancer Discov. 2019. PMID: 30266815 Free PMC article.
-
The development of a rapid patient-derived xenograft model to predict chemotherapeutic drug sensitivity/resistance in malignant glial tumors.Neuro Oncol. 2023 Sep 5;25(9):1605-1616. doi: 10.1093/neuonc/noad047. Neuro Oncol. 2023. PMID: 36821432 Free PMC article.
-
Establishment and characterization of patient-derived xenograft and its cell line of primary leiomyosarcoma of bone.In Vitro Cell Dev Biol Anim. 2018 Jun;54(6):458-467. doi: 10.1007/s11626-018-0258-2. Epub 2018 May 29. In Vitro Cell Dev Biol Anim. 2018. PMID: 29845452
-
Preclinical models for development of immune-oncology therapies.Immuno-oncol Insights. 2022;3(8):379-398. doi: 10.18609/ioi.2022.41. Epub 2022 Sep 26. Immuno-oncol Insights. 2022. PMID: 37132013 Free PMC article.
References
-
- Nielsen TO, West RB. Translating gene expression into clinical care: sarcomas as a paradigm. J Clin Oncol. 2010;28:1796–1805. - PubMed
-
- Nielsen TO, West RB, Linn SC, et al. Molecular characterisation of soft tissue tumors: a gene expression study. Lancet. 2002;359:1301–1307. - PubMed
-
- Baird K, Davis S, Antonescu CR, et al. Gene expression profiling of human sarcomas: insights into sarcoma biology. Cancer Res. 2005;65:9226–9235. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

