A pooled analysis of gemcitabine plus docetaxel versus capecitabine plus docetaxel in metastatic breast cancer
- PMID: 24705980
- PMCID: PMC4012969
- DOI: 10.1634/theoncologist.2013-0428
A pooled analysis of gemcitabine plus docetaxel versus capecitabine plus docetaxel in metastatic breast cancer
Abstract
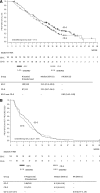
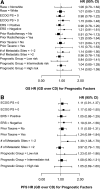
In two randomized phase III trials of patients with metastatic breast cancer (MBC), gemcitabine-docetaxel (GD) and capecitabine-docetaxel (CD) had similar efficacy, but distinct safety profiles. Methods. Data from two GD versus CD studies were pooled; overall survival (OS), progression-free survival (PFS), and overall response rate (ORR) were determined. Cox proportional hazards models identified prognostic factors associated with improved OS and PFS. Using a multivariate prognostic model incorporating identified adverse prognostic factors, we grouped MBC patients into low-, intermediate-, and high-risk categories. Hazard ratios (HRs) of GD over CD for OS and PFS were determined for subsets of patients. Results. Baseline demographics of the pooled population were mostly well balanced. In the pooled population, there were no significant differences between GD versus CD for OS (HR = 1.02; p = .824), PFS (HR = 1.15; p = .079), and ORR (p = .526). In the pooled crossover population, there were trends toward improved OS (HR = 0.82; p = .171) and PFS (HR = 0.93; p = .557) with GD. Several prognostic factors (including prior adjuvant taxane) for improved OS or PFS were identified; however, there were no significant interactions between treatment arms and prognostic factors for PFS or OS, except number of metastatic sites. In the prognostic model, median OS and PFS were numerically lower in the high-risk group versus the intermediate- and low-risk groups. Conclusion. This analysis confirms the lack of efficacy difference between GD and CD in the pooled population, crossover population, and almost all subpopulations. Several prognostic factors were associated with improved outcomes in the pooled population.
Keywords: Capecitabine; Docetaxel; Gemcitabine; Metastatic breast cancer; Pooled analysis.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures


Comment in
-
Adjuvant therapy-related shortening of survival (ATRESS): an underrated phenomenon.Oncologist. 2015 Jan;20(1):88. doi: 10.1634/theoncologist.2014-0273. Oncologist. 2015. PMID: 25589500 Free PMC article.
-
In reply.Oncologist. 2015 Jan;20(1):88. doi: 10.1634/theoncologist.2014-0284. Oncologist. 2015. PMID: 25589501 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Albain KS, de la Garza Salazar J, Pienkowski T, et al. Reducing the global breast cancer burden: The importance of patterns of care research. Clin Breast Cancer. 2005;6:412–420. - PubMed
-
- Hortobagyi GN, de la Garza Salazar J, Pritchard K, et al. The global breast cancer burden: Variations in epidemiology and survival. Clin Breast Cancer. 2005;6:391–401. - PubMed
-
- Mayer EL, Burstein HJ. Chemotherapy for metastatic breast cancer. Hematol Oncol Clin North Am. 2007;21:257–272. - PubMed
-
- Murphy CG, Seidman AD. Evolving approaches to metastatic breast cancer previously treated with anthracyclines and taxanes. Clin Breast Cancer. 2009;9(Suppl 2):S58–S65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

