Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy
- PMID: 24706013
- PMCID: PMC4013813
- DOI: 10.1212/WNL.0000000000000383
Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy
Abstract
Objective: To evaluate a trial of immunotherapy as an aid to diagnosis in suspected autoimmune epilepsy.
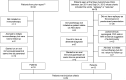
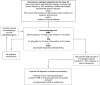
Method: We reviewed the charts of 110 patients seen at our autoimmune neurology clinic with seizures as a chief complaint. Twenty-nine patients met the following inclusion criteria: (1) autoimmune epilepsy suspected based on the presence of ≥ 1 neural autoantibody (n = 23), personal or family history or physical stigmata of autoimmunity, and frequent or medically intractable seizures; and (2) initiated a 6- to 12-week trial of IV methylprednisolone (IVMP), IV immune globulin (IVIg), or both. Patients were defined as responders if there was a 50% or greater reduction in seizure frequency.
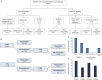
Results: Eighteen patients (62%) responded, of whom 10 (34%) became seizure-free; 52% improved with the first agent. Of those receiving a second agent after not responding to the first, 43% improved. A favorable response correlated with shorter interval between symptom onset and treatment initiation (median 9.5 vs 22 months; p = 0.048). Responders included 14/16 (87.5%) patients with antibodies to plasma membrane antigens, 2/6 (33%) patients seropositive for glutamic acid decarboxylase 65 antibodies, and 2/6 (33%) patients without detectable antibodies. Of 13 responders followed for more than 6 months after initiating long-term oral immunosuppression, response was sustained in 11 (85%).
Conclusions: These retrospective findings justify consideration of a trial of immunotherapy in patients with suspected autoimmune epilepsy.
Classification of evidence: This study provides Class IV evidence that in patients with suspected autoimmune epilepsy, IVMP, IVIg, or both improve seizure control.
Conflict of interest statement
M. Toledano and J. Britton report no disclosures relevant to the manuscript. A. McKeon receives research support from the Guthy-Jackson Charitable Foundation. C. Shin reports no disclosures relevant to the manuscript. V. Lennon is a named inventor on a patent (#7101679 issued 2006) relating to aquaporin-4 antibodies for diagnosis of neuromyelitis optica and receives royalties for this technology; is a named inventor on patents (#12/678,350 filed 2010 and #12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; and receives research support from the NIH (NS065829). A. Quek, E. So, and G. Worrell report no disclosures relevant to the manuscript. G. Cascino serves as associate editor of
Figures

Comment in
-
Immune therapy for pharmacoresistant epilepsy: ready to go?Neurology. 2014 May 6;82(18):1572-3. doi: 10.1212/WNL.0000000000000391. Epub 2014 Apr 4. Neurology. 2014. PMID: 24706017
References
-
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med 2011;365:919–926. - PubMed
-
- Bien CG, Urbach H, Schramm J, et al. . Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology 2007;69:1236–1244. - PubMed
-
- Hoffmann LA, Jarius S, Pellkofer HL, et al. . Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J Neurol Neurosurg Psychiatry 2008;79:767–773. - PubMed
-
- Vincent A, Buckley C, Schott JM, et al. . Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004;127:701–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
