Pulmonary retention of primed neutrophils: a novel protective host response, which is impaired in the acute respiratory distress syndrome
- PMID: 24706039
- PMCID: PMC4055272
- DOI: 10.1136/thoraxjnl-2013-204742
Pulmonary retention of primed neutrophils: a novel protective host response, which is impaired in the acute respiratory distress syndrome
Abstract
Rationale: Acute respiratory distress syndrome (ARDS) affects over 200000 people annually in the USA. Despite causing severe, and often refractory, hypoxaemia, the high mortality and long-term morbidity of ARDS results mainly from extra-pulmonary organ failure; however the mechanism for this organ crosstalk has not been determined.
Methods: Using autologous radiolabelled neutrophils we investigated the pulmonary transit of primed and unprimed neutrophils in humans. Flow cytometry of whole blood samples was used to assess transpulmonary neutrophil priming gradients in patients with ARDS, sepsis and perioperative controls.
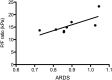
Main results: Unprimed neutrophils passed through the lungs with a transit time of 14.2 s, only 2.3 s slower than erythrocytes, and with <5% first-pass retention. Over 97% of neutrophils primed ex vivo with granulocyte macrophage colony-stimulating factor were retained on first pass, with 48% still remaining in the lungs at 40 min. Neutrophils exposed to platelet-activating factor were initially retained but subsequently released such that only 14% remained in the lungs at 40 min. Significant transpulmonary gradients of neutrophil CD62L cell surface expression were observed in ARDS compared with perioperative controls and patients with sepsis.
Conclusions: We demonstrated minimal delay and retention of unprimed neutrophils transiting the healthy human pulmonary vasculature, but marked retention of primed neutrophils; these latter cells then 'deprime' and are re-released into the systemic circulation. Further, we show that this physiological depriming mechanism may fail in patients with ARDS, resulting in increased numbers of primed neutrophils within the systemic circulation. This identifies a potential mechanism for the remote organ damage observed in patients with ARDS.
Keywords: ARDS; Neutrophil Biology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures



Comment in
-
Red, amber and green: the role of the lung in de-priming active systemic neutrophils.Thorax. 2014 Jul;69(7):606-8. doi: 10.1136/thoraxjnl-2014-205438. Thorax. 2014. PMID: 24917612 No abstract available.
Similar articles
-
Neutrophil sequestration in rat lungs.Thorax. 1995 Jun;50(6):661-7. doi: 10.1136/thx.50.6.661. Thorax. 1995. PMID: 7638810 Free PMC article.
-
Abnormal neutrophil-pulmonary interaction in the adult respiratory distress syndrome. Qualitative and quantitative assessment of pulmonary neutrophil kinetics in humans with in vivo 111indium neutrophil scintigraphy.Am Rev Respir Dis. 1986 May;133(5):797-804. Am Rev Respir Dis. 1986. PMID: 3706888
-
Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome.Crit Care Med. 2000 Jan;28(1):1-7. doi: 10.1097/00003246-200001000-00001. Crit Care Med. 2000. PMID: 10667491
-
The counter-intuitive role of the neutrophil in the acute respiratory distress syndrome.Br Med Bull. 2019 Sep 19;131(1):43-55. doi: 10.1093/bmb/ldz024. Br Med Bull. 2019. PMID: 31504234 Review.
-
The mercurial nature of neutrophils: still an enigma in ARDS?Am J Physiol Lung Cell Mol Physiol. 2014 Feb;306(3):L217-30. doi: 10.1152/ajplung.00311.2013. Epub 2013 Dec 6. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24318116 Free PMC article. Review.
Cited by
-
Neutrophil Extracellular Traps as Prognostic Markers in COVID-19: A Welcome Piece to the Puzzle.Arterioscler Thromb Vasc Biol. 2021 Feb;41(2):995-998. doi: 10.1161/ATVBAHA.120.315633. Epub 2021 Jan 27. Arterioscler Thromb Vasc Biol. 2021. PMID: 33955780 Free PMC article. No abstract available.
-
Bone Morphogenetic Protein 9 Enhances Lipopolysaccharide-Induced Leukocyte Recruitment to the Vascular Endothelium.J Immunol. 2016 Oct 15;197(8):3302-3314. doi: 10.4049/jimmunol.1601219. Epub 2016 Sep 19. J Immunol. 2016. PMID: 27647829 Free PMC article.
-
Myeloid Cells during Viral Infections and Inflammation.Viruses. 2019 Feb 19;11(2):168. doi: 10.3390/v11020168. Viruses. 2019. PMID: 30791481 Free PMC article. Review.
-
Targeting Neprilysin (NEP) pathways: A potential new hope to defeat COVID-19 ghost.Biochem Pharmacol. 2020 Aug;178:114057. doi: 10.1016/j.bcp.2020.114057. Epub 2020 May 27. Biochem Pharmacol. 2020. PMID: 32470547 Free PMC article. Review.
-
Neutrophil Dysfunction in the Airways of Children with Acute Respiratory Failure Due to Lower Respiratory Tract Viral and Bacterial Coinfections.Sci Rep. 2019 Feb 27;9(1):2874. doi: 10.1038/s41598-019-39726-w. Sci Rep. 2019. PMID: 30814584 Free PMC article. Clinical Trial.
References
-
- Rubenfeld GD, Cauldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Eng J Med 2005;353:1685–93 - PubMed
-
- Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–304 - PubMed
-
- Hopkins RO, Weaver LK, Collingridge D, et al. Two-tear cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005;171:340–7 - PubMed
-
- Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003;289:2104–12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
