Surface modification of a polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU) nanocomposite polymer as a stent coating for enhanced capture of endothelial progenitor cells
- PMID: 24706135
- PMCID: PMC3979469
- DOI: 10.1186/1559-4106-8-23
Surface modification of a polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU) nanocomposite polymer as a stent coating for enhanced capture of endothelial progenitor cells
Abstract
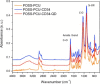
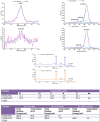
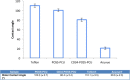
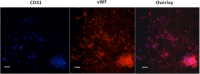
An unmet need exists for the development of next-generation multifunctional nanocomposite materials for biomedical applications, particularly in the field of cardiovascular regenerative biology. Herein, we describe the preparation and characterization of a novel polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU) nanocomposite polymer with covalently attached anti-CD34 antibodies to enhance capture of circulating endothelial progenitor cells (EPC). This material may be used as a new coating for bare metal stents used after balloon angioplasty to improve re-endothelialization. Biophysical characterization techniques were used to assess POSS-PCU and its subsequent functionalization with anti-CD34 antibodies. Results indicated successful covalent attachment of anti-CD34 antibodies on the surface of POSS-PCU leading to an increased propensity for EPC capture, whilst maintaining in vitro biocompatibility and hemocompatibility. POSS-PCU has already been used in 3 first-in-man studies, as a bypass graft, lacrimal duct and a bioartificial trachea. We therefore postulate that its superior biocompatibility and unique biophysical properties would render it an ideal candidate for coating medical devices, with stents as a prime example. Taken together, anti-CD34 functionalized POSS-PCU could form the basis of a nano-inspired polymer platform for the next generation stent coatings.
Figures












Similar articles
-
An anti-CD34 antibody-functionalized clinical-grade POSS-PCU nanocomposite polymer for cardiovascular stent coating applications: a preliminary assessment of endothelial progenitor cell capture and hemocompatibility.PLoS One. 2013 Oct 8;8(10):e77112. doi: 10.1371/journal.pone.0077112. eCollection 2013. PLoS One. 2013. PMID: 24116210 Free PMC article.
-
Polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU): applications in nanotechnology and regenerative medicine.Crit Rev Biomed Eng. 2013;41(6):495-513. Crit Rev Biomed Eng. 2013. PMID: 24940662 Review.
-
Novel heart valve prosthesis with self-endothelialization potential made of modified polyhedral oligomeric silsesquioxane-nanocomposite material.Biointerphases. 2016 Jun 13;11(2):029801. doi: 10.1116/1.4939036. Biointerphases. 2016. PMID: 26763768
-
Synergistic photothermal ablative effects of functionalizing carbon nanotubes with a POSS-PCU nanocomposite polymer.J Nanobiotechnology. 2012 Jul 31;10:34. doi: 10.1186/1477-3155-10-34. J Nanobiotechnology. 2012. PMID: 22849373 Free PMC article.
-
Recent Advances in Polyurethane/POSS Hybrids for Biomedical Applications.Molecules. 2021 Dec 22;27(1):40. doi: 10.3390/molecules27010040. Molecules. 2021. PMID: 35011280 Free PMC article. Review.
Cited by
-
Biological Effects, Applications and Design Strategies of Medical Polyurethanes Modified by Nanomaterials.Int J Nanomedicine. 2022 Dec 29;17:6791-6819. doi: 10.2147/IJN.S393207. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36600880 Free PMC article. Review.
-
Electrospun Poly(carbonate-urea-urethane)s Nonwovens with Shape-Memory Properties as a Potential Biomaterial.ACS Biomater Sci Eng. 2023 Dec 11;9(12):6683-6697. doi: 10.1021/acsbiomaterials.3c01214. Epub 2023 Nov 30. ACS Biomater Sci Eng. 2023. PMID: 38032398 Free PMC article.
-
Surface engineering at the nanoscale: A way forward to improve coronary stent efficacy.APL Bioeng. 2021 Jun 1;5(2):021508. doi: 10.1063/5.0037298. eCollection 2021 Jun. APL Bioeng. 2021. PMID: 34104846 Free PMC article. Review.
-
Nanohydroxyapatite Effect on the Degradation, Osteoconduction and Mechanical Properties of Polymeric Bone Tissue Engineered Scaffolds.Open Orthop J. 2016 Dec 30;10:900-919. doi: 10.2174/1874325001610010900. eCollection 2016. Open Orthop J. 2016. PMID: 28217213 Free PMC article.
-
Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices.Int J Mol Sci. 2023 Feb 16;24(4):3963. doi: 10.3390/ijms24043963. Int J Mol Sci. 2023. PMID: 36835389 Free PMC article. Review.
References
-
- Cardiovascular Diseases http://www.who.int/mediacentre/factsheets/fs317/en/index.html
-
- Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, O’Shaughnessy CD, DeMaio S, Hall P and Popma JJ, Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease, JAMA: the journal of the American Medical Association 294(10), 1215 (2005)10.1001/jama.294.10.1215. - DOI - PubMed
-
- Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jüni P, Sianos G and Hellige Get al., Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study, Lancet 369(9562), 667 (2007)10.1016/S0140-6736(07)60314-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

