Genetic heterogeneity beyond CYP2C8*3 does not explain differential sensitivity to paclitaxel-induced neuropathy
- PMID: 24706167
- PMCID: PMC4256153
- DOI: 10.1007/s10549-014-2910-1
Genetic heterogeneity beyond CYP2C8*3 does not explain differential sensitivity to paclitaxel-induced neuropathy
Abstract
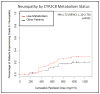
The development of paclitaxel-induced peripheral neuropathy (PIPN) is influenced by drug exposure and patient genetics. The purpose of this analysis was to expand on a previous reported association of CYP2C8*3 and PIPN risk by investigating additional polymorphisms in CYP2C8 and in hundreds of other genes potentially relevant to paclitaxel pharmacokinetics. Clinical data was collected prospectively in an observational registry of newly diagnosed breast cancer patients. Patients treated with paclitaxel-containing regimens were genotyped using the Affymetrix DMET™ Plus chip. Patients who carried the CYP2C8*2, *3, or *4 variant were collapsed into a low-metabolizer CYP2C8 phenotype for association with PIPN. Separately, all SNPs that surpassed quality control were assessed individually and as a composite of genetic ancestry for associations with PIPN. 412 paclitaxel-treated patients and 564 genetic markers were included in the analysis. The risk of PIPN was significantly greater in the CYP2C8 low-metabolizer group (HR = 1.722, p = 0.018); however, the influences of the *2 and *4 SNPs were not independently significant (*2: p = 0.847, *4: p = 0.408). One intronic SNP in ABCG1 (rs492338) surpassed the exploratory significance threshold for an association with PIPN in the Caucasian cohort (p = 0.0008) but not in the non-Caucasian replication group (p = 0.54). Substantial genetic variability was observed within self-reported racial groups but this genetic variability was not associated with risk of grade 2+ PIPN. The pharmacogenetic heterogeneity within a cohort of breast cancer patients is dramatic, though we did not find evidence that this heterogeneity directly influences the risk of PIPN beyond the contribution of CYP2C8*3.
Conflict of interest statement
Conflicts of Interest:
L. Scott Clark is an employee of Gentris Corp. Howard L. McLeod and Alison A. Motsinger-Reif were consultants for Gentris Corp.
Figures


References
-
- Diéras V, Fumoleau P, Romieu G, Tubiana-Hulin M, Namer M, Mauriac L, et al. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol. 2004 Dec 15;22(24):4958–65. - PubMed
-
- Martin M, Rodriguez-Lescure Ã, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008 Jun 04;100(11):805–14. - PubMed
-
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003 Mar 15;21(6):976–83. - PubMed
-
- Freilich RJ, Balmaceda C, Seidman AD, Rubin M, DeAngelis LM. Motor neuropathy due to docetaxel and paclitaxel. Neurology. 1996 Jul 01;47(1):115–8. - PubMed
-
- Mielke S, Mross K, Gerds TA, Schmidt A, Wasch R, Berger DP, et al. Comparative neurotoxicity of weekly non-break paclitaxel infusions over 1 versus 3 h. Anticancer Drugs. 2003;14(10):785–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

