Heat shock protein 90 controls HIV-1 reactivation from latency
- PMID: 24706778
- PMCID: PMC3992654
- DOI: 10.1073/pnas.1320178111
Heat shock protein 90 controls HIV-1 reactivation from latency
Abstract
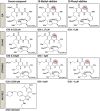
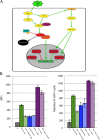
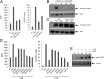
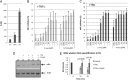
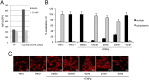
Latency allows HIV-1 to persist in long-lived cellular reservoirs, preventing virus eradication. We have previously shown that the heat shock protein 90 (Hsp90) is required for HIV-1 gene expression and mediates greater HIV-1 replication in conditions of hyperthermia. Here we report that specific inhibitors of Hsp90 such as 17-(N-allylamino)-17-demethoxygeldanamycin and AUY922 prevent HIV-1 reactivation in CD4+ T cells. A single modification at position 19 in the Hsp90 inhibitors abolished this activity, supporting the specificity of the target. We tested the impact of Hsp90 on known pathways involved in HIV-1 reactivation from latency; they include protein kinase Cs(PKCs), mitogen activated protein kinase/extracellular signal regulated kinase/positive transcriptional elongation factor-b and NF-κB. We found that Hsp90 was required downstream of PKCs and was not required for mitogen activated protein kinase activation. Inhibition of Hsp90 reduced degradation of IkBα and blocked nuclear translocation of transcription factor p65/p50, suppressing the NF-κB pathway. Coimmunoprecipitation experiments showed that Hsp90 interacts with inhibitor of nuclear factor kappa-B kinase (IKK) together with cochaperone Cdc37, which is critical for the activity of several kinases. Targeting of Hsp90 by AUY922 dissociated Cdc37 from the complex. Therefore, Hsp90 controls HIV-1 reactivation from latency by keeping the IKK complex functional and thus connects T-cell activation with HIV-1 replication. AUY922 is in phase II clinical trial and, in combination with a PKC-ϑ inhibitor in phase II clinical trial, almost completely suppressed HIV-1 reactivation at 15 nM with no cytotoxicity. Selective targeting of the Hsp90/Cdc37 interaction may provide a powerful approach to suppress HIV-1 reactivation from latency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Palella FJ, Jr, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. - PubMed
-
- Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–1300. - PubMed
-
- Ramratnam B, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6(1):82–85. - PubMed
-
- Buzón MJ, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

