Differential ubiquitination and degradation of huntingtin fragments modulated by ubiquitin-protein ligase E3A
- PMID: 24706802
- PMCID: PMC3992696
- DOI: 10.1073/pnas.1402215111
Differential ubiquitination and degradation of huntingtin fragments modulated by ubiquitin-protein ligase E3A
Abstract
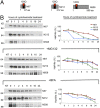
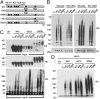
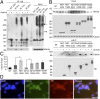
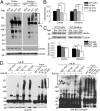
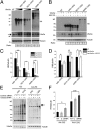
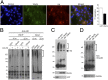
Ubiquitination of misfolded proteins, a common feature of many neurodegenerative diseases, is mediated by different lysine (K) residues in ubiquitin and alters the levels of toxic proteins. In Huntington disease, polyglutamine expansion causes N-terminal huntingtin (Htt) to misfold, inducing neurodegeneration. Here we report that shorter N-terminal Htt fragments are more stable than longer fragments and find differential ubiquitination via K63 of ubiquitin. Aging decreases proteasome-mediated Htt degradation, at the same time increasing K63-mediated ubiquitination and subsequent Htt aggregation in HD knock-in mice. The association of Htt with the K48-specific E3 ligase, Ube3a, is decreased in aged mouse brain. Overexpression of Ube3a in HD mouse brain reduces K63-mediated ubiquitination and Htt aggregation, enhancing its degradation via the K48 ubiquitin-proteasome system. Our findings suggest that aging-dependent Ube3a levels result in differential ubiquitination and degradation of Htt fragments, thereby contributing to the age-related neurotoxicity of mutant Htt.
Keywords: misfolding; proteolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gusella JF, MacDonald ME, Ambrose CM, Duyao MP. Molecular genetics of Huntington’s disease. Arch Neurol. 1993;50(11):1157–1163. - PubMed
-
- Snell RG, et al. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington’s disease. Nat Genet. 1993;4(4):393–397. - PubMed
-
- Kuemmerle S, et al. Huntington aggregates may not predict neuronal death in Huntington’s disease. Ann Neurol. 1999;46(6):842–849. - PubMed
-
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57(5):369–384. - PubMed
-
- DiFiglia M, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277(5334):1990–1993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

