Acentriolar mitosis activates a p53-dependent apoptosis pathway in the mouse embryo
- PMID: 24706806
- PMCID: PMC3992648
- DOI: 10.1073/pnas.1400568111
Acentriolar mitosis activates a p53-dependent apoptosis pathway in the mouse embryo
Abstract
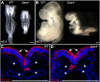
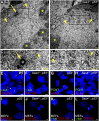
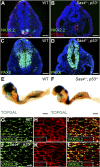
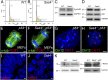
Centrosomes are the microtubule-organizing centers of animal cells that organize interphase microtubules and mitotic spindles. Centrioles are the microtubule-based structures that organize centrosomes, and a defined set of proteins, including spindle assembly defective-4 (SAS4) (CPAP/CENPJ), is required for centriole biogenesis. The biological functions of centrioles and centrosomes vary among animals, and the functions of mammalian centrosomes have not been genetically defined. Here we use a null mutation in mouse Sas4 to define the cellular and developmental functions of mammalian centrioles in vivo. Sas4-null embryos lack centrosomes but survive until midgestation. As expected, Sas4(-/-) mutants lack primary cilia and therefore cannot respond to Hedgehog signals, but other developmental signaling pathways are normal in the mutants. Unlike mutants that lack cilia, Sas4(-/-) embryos show widespread apoptosis associated with global elevated expression of p53. Cell death is rescued in Sas4(-/-) p53(-/-) double-mutant embryos, demonstrating that mammalian centrioles prevent activation of a p53-dependent apoptotic pathway. Expression of p53 is not activated by abnormalities in bipolar spindle organization, chromosome segregation, cell-cycle profile, or DNA damage response, which are normal in Sas4(-/-) mutants. Instead, live imaging shows that the duration of prometaphase is prolonged in the mutants while two acentriolar spindle poles are assembled. Independent experiments show that prolonging spindle assembly is sufficient to trigger p53-dependent apoptosis. We conclude that a short delay in the prometaphase caused by the absence of centrioles activates a previously undescribed p53-dependent cell death pathway in the rapidly dividing cells of the mouse embryo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J Cell Sci. 2008;121(Suppl 1):1–84. - PubMed
-
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: Centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8(6):451–463. - PubMed
-
- Kirkham M, Müller-Reichert T, Oegema K, Grill S, Hyman AA. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell. 2003;112(4):575–587. - PubMed
-
- Leidel S, Gönczy P. SAS-4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Dev Cell. 2003;4(3):431–439. - PubMed
-
- Basto R, et al. Flies without centrioles. Cell. 2006;125(7):1375–1386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

