Solving a Levinthal's paradox for virus assembly identifies a unique antiviral strategy
- PMID: 24706827
- PMCID: PMC3986145
- DOI: 10.1073/pnas.1319479111
Solving a Levinthal's paradox for virus assembly identifies a unique antiviral strategy
Abstract
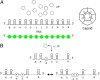
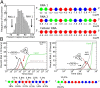
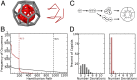
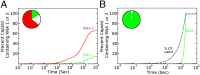
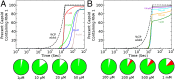
One of the important puzzles in virology is how viruses assemble the protein containers that package their genomes rapidly and efficiently in vivo while avoiding triggering their hosts' antiviral defenses. Viral assembly appears directed toward a relatively small subset of the vast number of all possible assembly intermediates and pathways, akin to Levinthal's paradox for the folding of polypeptide chains. Using an in silico assembly model, we demonstrate that this reduction in complexity can be understood if aspects of in vivo assembly, which have mostly been neglected in in vitro experimental and theoretical modeling assembly studies, are included in the analysis. In particular, we show that the increasing viral coat protein concentration that occurs in infected cells plays unexpected and vital roles in avoiding potential kinetic assembly traps, significantly reducing the number of assembly pathways and assembly initiation sites, and resulting in enhanced assembly efficiency and genome packaging specificity. Because capsid assembly is a vital determinant of the overall fitness of a virus in the infection process, these insights have important consequences for our understanding of how selection impacts on the evolution of viral quasispecies. These results moreover suggest strategies for optimizing the production of protein nanocontainers for drug delivery and of virus-like particles for vaccination. We demonstrate here in silico that drugs targeting the specific RNA-capsid protein contacts can delay assembly, reduce viral load, and lead to an increase of misencapsidation of cellular RNAs, hence opening up unique avenues for antiviral therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

