Glycogen synthase kinase 3 inhibitors induce the canonical WNT/β-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma
- PMID: 24706870
- PMCID: PMC3986146
- DOI: 10.1073/pnas.1317731111
Glycogen synthase kinase 3 inhibitors induce the canonical WNT/β-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma
Abstract
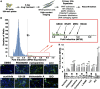
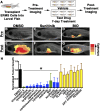
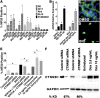
Embryonal rhabdomyosarcoma (ERMS) is a common pediatric malignancy of muscle, with relapse being the major clinical challenge. Self-renewing tumor-propagating cells (TPCs) drive cancer relapse and are confined to a molecularly definable subset of ERMS cells. To identify drugs that suppress ERMS self-renewal and induce differentiation of TPCs, a large-scale chemical screen was completed. Glycogen synthase kinase 3 (GSK3) inhibitors were identified as potent suppressors of ERMS growth through inhibiting proliferation and inducing terminal differentiation of TPCs into myosin-expressing cells. In support of GSK3 inhibitors functioning through activation of the canonical WNT/β-catenin pathway, recombinant WNT3A and stabilized β-catenin also enhanced terminal differentiation of human ERMS cells. Treatment of ERMS-bearing zebrafish with GSK3 inhibitors activated the WNT/β-catenin pathway, resulting in suppressed ERMS growth, depleted TPCs, and diminished self-renewal capacity in vivo. Activation of the canonical WNT/β-catenin pathway also significantly reduced self-renewal of human ERMS, indicating a conserved function for this pathway in modulating ERMS self-renewal. In total, we have identified an unconventional tumor suppressive role for the canonical WNT/β-catenin pathway in regulating self-renewal of ERMS and revealed therapeutic strategies to target differentiation of TPCs in ERMS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Basic research: Rebooting rhabdomyosarcoma's operating system.Nat Rev Clin Oncol. 2014 May;11(5):242. doi: 10.1038/nrclinonc.2014.62. Epub 2014 Apr 15. Nat Rev Clin Oncol. 2014. PMID: 24732942 No abstract available.
References
-
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–284. - PubMed
-
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. - PubMed
-
- Uckun FM, et al. Leukemic cell growth in SCID mice as a predictor of relapse in high-risk B-lineage acute lymphoblastic leukemia. Blood. 1995;85(4):873–878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21CA156056/CA/NCI NIH HHS/United States
- K08AR063165/AR/NIAMS NIH HHS/United States
- R01CA154923/CA/NCI NIH HHS/United States
- R01 CA154923/CA/NCI NIH HHS/United States
- K99CA175184/CA/NCI NIH HHS/United States
- R21 CA156056/CA/NCI NIH HHS/United States
- K08 AR063165/AR/NIAMS NIH HHS/United States
- U54CA168512/CA/NCI NIH HHS/United States
- R01CA143082/CA/NCI NIH HHS/United States
- R00 CA175184/CA/NCI NIH HHS/United States
- R01 CA143082/CA/NCI NIH HHS/United States
- K99 CA175184/CA/NCI NIH HHS/United States
- U54 CA168512/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

