Crystal structure of PhnZ in complex with substrate reveals a di-iron oxygenase mechanism for catabolism of organophosphonates
- PMID: 24706911
- PMCID: PMC3986159
- DOI: 10.1073/pnas.1320039111
Crystal structure of PhnZ in complex with substrate reveals a di-iron oxygenase mechanism for catabolism of organophosphonates
Erratum in
- Proc Natl Acad Sci U S A. 2014 Jun 3;111(22)8311
Abstract
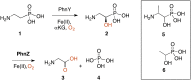
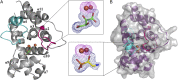
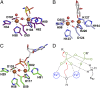
The enzymes PhnY and PhnZ comprise an oxidative catabolic pathway that enables marine bacteria to use 2-aminoethylphosphonic acid as a source of inorganic phosphate. PhnZ is notable for catalyzing the oxidative cleavage of a carbon-phosphorus bond using Fe(II) and dioxygen, despite belonging to a large family of hydrolytic enzymes, the HD-phosphohydrolase superfamily. We have determined high-resolution structures of PhnZ bound to its substrate, (R)-2-amino-1-hydroxyethylphosphonate (2.1 Å), and a buffer additive, l-tartrate (1.7 Å). The structures reveal PhnZ to have an active site containing two Fe ions coordinated by four histidines and two aspartates that is strikingly similar to the carbon-carbon bond cleaving enzyme, myo-inositol-oxygenase. The exception is Y24, which forms a transient ligand interaction at the dioxygen binding site of Fe2. Site-directed mutagenesis and kinetic analysis with substrate analogs revealed the roles of key active site residues. A fifth histidine that is conserved in the PhnZ subclade, H62, specifically interacts with the substrate 1-hydroxyl. The structures also revealed that Y24 and E27 mediate a unique induced-fit mechanism whereby E27 specifically recognizes the 2-amino group of the bound substrate and toggles the release of Y24 from the active site, thereby creating space for molecular oxygen to bind to Fe2. Structural comparisons of PhnZ reveal an evolutionary connection between Fe(II)-dependent hydrolysis of phosphate esters and oxidative carbon-phosphorus or carbon-carbon bond cleavage, thus uniting the diverse chemistries that are found in the HD superfamily.
Keywords: C–H bond activation; C–P bond cleavage; nonheme iron-dependent oxygenase; phosphonate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

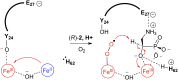
Similar articles
-
C-H Bond Cleavage Is Rate-Limiting for Oxidative C-P Bond Cleavage by the Mixed Valence Diiron-Dependent Oxygenase PhnZ.Biochemistry. 2019 Dec 31;58(52):5271-5280. doi: 10.1021/acs.biochem.9b00145. Epub 2019 May 10. Biochemistry. 2019. PMID: 31046250 Free PMC article.
-
Organophosphonate-degrading PhnZ reveals an emerging family of HD domain mixed-valent diiron oxygenases.Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):18874-9. doi: 10.1073/pnas.1315927110. Epub 2013 Nov 6. Proc Natl Acad Sci U S A. 2013. PMID: 24198335 Free PMC article.
-
PhnY and PhnZ comprise a new oxidative pathway for enzymatic cleavage of a carbon-phosphorus bond.J Am Chem Soc. 2012 May 23;134(20):8364-7. doi: 10.1021/ja302072f. Epub 2012 May 11. J Am Chem Soc. 2012. PMID: 22564006
-
Phosphonate biosynthesis and catabolism: a treasure trove of unusual enzymology.Curr Opin Chem Biol. 2013 Aug;17(4):580-8. doi: 10.1016/j.cbpa.2013.06.018. Epub 2013 Jul 17. Curr Opin Chem Biol. 2013. PMID: 23870698 Free PMC article. Review.
-
Variations of the 2-His-1-carboxylate theme in mononuclear non-heme FeII oxygenases.Chembiochem. 2006 Oct;7(10):1536-48. doi: 10.1002/cbic.200600152. Chembiochem. 2006. PMID: 16858718 Review.
Cited by
-
The HD-Domain Metalloprotein Superfamily: An Apparent Common Protein Scaffold with Diverse Chemistries.Catalysts. 2020 Oct;10(10):1191. doi: 10.3390/catal10101191. Epub 2020 Oct 15. Catalysts. 2020. PMID: 34094591 Free PMC article.
-
Deciphering the role of recurrent FAD-dependent enzymes in bacterial phosphonate catabolism.iScience. 2023 Oct 4;26(11):108108. doi: 10.1016/j.isci.2023.108108. eCollection 2023 Nov 17. iScience. 2023. PMID: 37876809 Free PMC article.
-
An HD domain phosphohydrolase active site tailored for oxetanocin-A biosynthesis.Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):13750-13755. doi: 10.1073/pnas.1613610113. Epub 2016 Nov 14. Proc Natl Acad Sci U S A. 2016. PMID: 27849620 Free PMC article.
-
PcxL and HpxL are flavin-dependent, oxime-forming N-oxidases in phosphonocystoximic acid biosynthesis in Streptomyces.J Biol Chem. 2018 May 4;293(18):6859-6868. doi: 10.1074/jbc.RA118.001721. Epub 2018 Mar 14. J Biol Chem. 2018. PMID: 29540479 Free PMC article.
-
The Stereochemical Course of the α-Hydroxyphosphonate-Phosphate Rearrangement.Chemistry. 2015 Jul 6;21(28):10200-6. doi: 10.1002/chem.201406661. Epub 2015 Jun 8. Chemistry. 2015. PMID: 26059025 Free PMC article.
References
-
- Dyhrman ST, Benitez-Nelson CR, Orchard ED, Haley ST, Pellechia PJ. A microbial source of phosphonates in oligotrophic marine systems. Nat Geosci. 2009;2(10):696–699.
-
- Dyhrman ST, et al. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature. 2006;439(7072):68–71. - PubMed
-
- Hudson JJ, Taylor WD, Schindler DW. Phosphate concentrations in lakes. Nature. 2000;406(6791):54–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

