Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle
- PMID: 24706914
- PMCID: PMC3986127
- DOI: 10.1073/pnas.1403576111
Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle
Abstract
Previous (13)C magnetic resonance spectroscopy experiments have shown that over a wide range of neuronal activity, approximately one molecule of glucose is oxidized for every molecule of glutamate released by neurons and recycled through astrocytic glutamine. The measured kinetics were shown to agree with the stoichiometry of a hypothetical astrocyte-to-neuron lactate shuttle model, which predicted negligible functional neuronal uptake of glucose. To test this model, we measured the uptake and phosphorylation of glucose in nerve terminals isolated from rats infused with the glucose analog, 2-fluoro-2-deoxy-D-glucose (FDG) in vivo. The concentrations of phosphorylated FDG (FDG6P), normalized with respect to known neuronal metabolites, were compared in nerve terminals, homogenate, and cortex of anesthetized rats with and without bicuculline-induced seizures. The increase in FDG6P in nerve terminals agreed well with the increase in cortical neuronal glucose oxidation measured previously under the same conditions in vivo, indicating that direct uptake and oxidation of glucose in nerve terminals is substantial under resting and activated conditions. These results suggest that neuronal glucose-derived pyruvate is the major oxidative fuel for activated neurons, not lactate-derived from astrocytes, contradicting predictions of the original astrocyte-to-neuron lactate shuttle model under the range of study conditions.
Keywords: 2-fluorodeoxyglucose; glutamate-glutamine cycle; neuroenergetics; neuronal glucose phosphorylation; synaptoneurosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
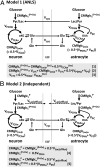
 . Predicted rates of CMRglcn(P+Ox) for model 1 were calculated using Eq. 1, shown at the bottom of A. In the revised description of model 1 by Hyder et al. (3), glucose phosphorylation in neurons above isoelectricity can occur depending on the magnitude of astroglial oxidation and its dependence on neural activity. (B) Independent-type model (model 2) in which neurons and astrocytes take up and oxidize glucose according to their respective energy needs. Phosphorylated glucose not oxidized within the cell may be effluxed as lactate, VLac(efflux), which is shown by dashed lines, reflecting uncertainty of the lactate-releasing neural cells (13, 41). Predicted rates of
. Predicted rates of CMRglcn(P+Ox) for model 1 were calculated using Eq. 1, shown at the bottom of A. In the revised description of model 1 by Hyder et al. (3), glucose phosphorylation in neurons above isoelectricity can occur depending on the magnitude of astroglial oxidation and its dependence on neural activity. (B) Independent-type model (model 2) in which neurons and astrocytes take up and oxidize glucose according to their respective energy needs. Phosphorylated glucose not oxidized within the cell may be effluxed as lactate, VLac(efflux), which is shown by dashed lines, reflecting uncertainty of the lactate-releasing neural cells (13, 41). Predicted rates of  for model 2 were calculated using Eq. 4, shown at the bottom of B.
for model 2 were calculated using Eq. 4, shown at the bottom of B.  , rate of glucose oxidation in astrocytes
, rate of glucose oxidation in astrocytes  ;
;  , rate of glucose oxidized in neurons at isoelectricity
, rate of glucose oxidized in neurons at isoelectricity  ;
;  , rate of total glucose phosphorylation in astrocytes, which includes oxidative and nonoxidative (net lactate efflux) catabolism;
, rate of total glucose phosphorylation in astrocytes, which includes oxidative and nonoxidative (net lactate efflux) catabolism;  , rate of glucose phosphorylation in astrocytes with oxidation, includes lactate efflux to neurons at the rate Vcyc (model 1) or to extracellular fluid (model 2);
, rate of glucose phosphorylation in astrocytes with oxidation, includes lactate efflux to neurons at the rate Vcyc (model 1) or to extracellular fluid (model 2);  , rate of total glucose phosphorylation in neurons, which includes oxidative and nonoxidative (lactate efflux) catabolism;
, rate of total glucose phosphorylation in neurons, which includes oxidative and nonoxidative (lactate efflux) catabolism;  , rate of glucose phosphorylation in neurons with oxidation; Gln, glutamine; Glu, glutamate; Lac, lactate; OAA, oxaloacetate; Pyr, pyruvate; αKG, α-ketoglutarate; VPC, pyruvate carboxylase rate in astrocytes; VPDHa, pyruvate dehydrogenase rate in astrocytes; VTCAa, TCA cycle flux in astrocytes; VTCAn, TCA cycle flux in neurons
, rate of glucose phosphorylation in neurons with oxidation; Gln, glutamine; Glu, glutamate; Lac, lactate; OAA, oxaloacetate; Pyr, pyruvate; αKG, α-ketoglutarate; VPC, pyruvate carboxylase rate in astrocytes; VPDHa, pyruvate dehydrogenase rate in astrocytes; VTCAa, TCA cycle flux in astrocytes; VTCAn, TCA cycle flux in neurons  ;
;  , TCA cycle flux in neurons at isoelectricity.
, TCA cycle flux in neurons at isoelectricity.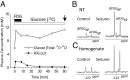
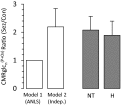
 , were calculated using Eqs. 1−3 (model 1) and Eqs. 4 and 5 (model 2) for control and seizure conditions and expressed as a ratio.
, were calculated using Eqs. 1−3 (model 1) and Eqs. 4 and 5 (model 2) for control and seizure conditions and expressed as a ratio.References
-
- Hyder F, et al. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26(7):865–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

