Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis
- PMID: 24706917
- PMCID: PMC3992636
- DOI: 10.1073/pnas.1402218111
Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis
Abstract
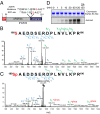
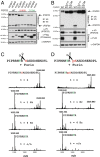
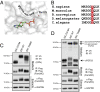
The family with sequence similarity 20, member C (Fam20C) has recently been identified as the Golgi casein kinase. Fam20C phosphorylates secreted proteins on Ser-x-Glu/pSer motifs and loss-of-function mutations in the kinase cause Raine syndrome, an often-fatal osteosclerotic bone dysplasia. Fam20C is potentially an upstream regulator of the phosphate-regulating hormone fibroblast growth factor 23 (FGF23), because humans with FAM20C mutations and Fam20C KO mice develop hypophosphatemia due to an increase in full-length, biologically active FGF23. However, the mechanism by which Fam20C regulates FGF23 is unknown. Here we show that Fam20C directly phosphorylates FGF23 on Ser(180), within the FGF23 R(176)XXR(179)/S(180)AE subtilisin-like proprotein convertase motif. This phosphorylation event inhibits O-glycosylation of FGF23 by polypeptide N-acetylgalactosaminyltransferase 3 (GalNAc-T3), and promotes FGF23 cleavage and inactivation by the subtilisin-like proprotein convertase furin. Collectively, our results provide a molecular mechanism by which FGF23 is dynamically regulated by phosphorylation, glycosylation, and proteolysis. Furthermore, our findings suggest that cross-talk between phosphorylation and O-glycosylation of proteins in the secretory pathway may be an important mechanism by which secreted proteins are regulated.
Keywords: Fam20; chronic kidney disease; familial tumoral calcinosis; phosphate homeostasis; rickets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

