Activation of estrogen receptor α enhances bradykinin signaling in peripheral sensory neurons of female rats
- PMID: 24706985
- PMCID: PMC4019325
- DOI: 10.1124/jpet.114.212977
Activation of estrogen receptor α enhances bradykinin signaling in peripheral sensory neurons of female rats
Abstract
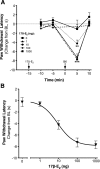
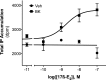
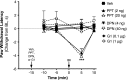
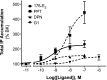
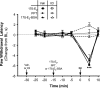
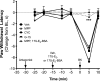
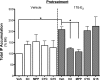
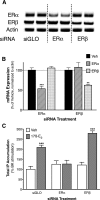
Numerous studies have demonstrated that females have a higher risk of experiencing several pain disorders with either greater frequency or severity than males. Although the mechanisms that underlie this sex disparity remain unclear, several studies have shown an important role for sex steroids, such as estrogen, in the modulation of nociception. Receptors for estrogen are present in primary afferent neurons in the trigeminal and dorsal root ganglia, and brief exposure to estrogen increases responses to the inflammatory mediator bradykinin (BK). However, the mechanism for estrogen-mediated enhancement of BK signaling is not fully understood. The aim of the present study was to evaluate the relative contributions of estrogen receptor α (ERα), ERβ, and G protein-coupled estrogen receptor 1 (GPER) to the enhanced signaling of the inflammatory mediator BK by 17β-estradiol (17β-E2) in primary sensory neurons from female rats in culture (ex vivo) and in behavioral assays of nociception in vivo. The effects of 17β-E2 on BK responses were mimicked by ERα-selective agonists and blocked by ERα-selective antagonists and by small interfering RNA knockdown of ERα. The data indicate that ERα is required for 17β-E2-mediated enhancement of BK signaling in peripheral sensory neurons in female rats.
Figures
Similar articles
-
17beta-estradiol rapidly enhances bradykinin signaling in primary sensory neurons in vitro and in vivo.J Pharmacol Exp Ther. 2010 Oct;335(1):190-6. doi: 10.1124/jpet.110.167445. Epub 2010 Jul 20. J Pharmacol Exp Ther. 2010. PMID: 20647494 Free PMC article.
-
Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TMJ.PLoS One. 2017 Jun 5;12(6):e0178589. doi: 10.1371/journal.pone.0178589. eCollection 2017. PLoS One. 2017. PMID: 28582470 Free PMC article.
-
17β-Estradiol Enhances ASIC Activity in Primary Sensory Neurons to Produce Sex Difference in Acidosis-Induced Nociception.Endocrinology. 2015 Dec;156(12):4660-71. doi: 10.1210/en.2015-1557. Epub 2015 Oct 6. Endocrinology. 2015. PMID: 26441237
-
17β-Estradiol and Agonism of G-protein-Coupled Estrogen Receptor Enhance Hippocampal Memory via Different Cell-Signaling Mechanisms.J Neurosci. 2016 Mar 16;36(11):3309-21. doi: 10.1523/JNEUROSCI.0257-15.2016. J Neurosci. 2016. PMID: 26985039 Free PMC article.
-
Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: effects on intracellular molecular targets and antioxidant enzymes.Mol Immunol. 2013 Dec;56(4):328-39. doi: 10.1016/j.molimm.2013.05.226. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911387
Cited by
-
Pirt contributes to uterine contraction-induced pain in mice.Mol Pain. 2015 Sep 17;11:57. doi: 10.1186/s12990-015-0054-x. Mol Pain. 2015. PMID: 26376721 Free PMC article.
-
G protein-coupled receptor kinase 2 (GRK2) as a multifunctional signaling hub.Cell Mol Life Sci. 2019 Nov;76(22):4423-4446. doi: 10.1007/s00018-019-03274-3. Epub 2019 Aug 20. Cell Mol Life Sci. 2019. PMID: 31432234 Free PMC article. Review.
-
Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis.Am J Pathol. 2015 Aug;185(8):2286-97. doi: 10.1016/j.ajpath.2015.04.012. Epub 2015 Jun 12. Am J Pathol. 2015. PMID: 26073038 Free PMC article.
-
Estrogen modulation of the pronociceptive effects of serotonin on female rat trigeminal sensory neurons is timing dependent and dosage dependent and requires estrogen receptor alpha.Pain. 2022 Aug 1;163(8):e899-e916. doi: 10.1097/j.pain.0000000000002604. Epub 2022 Feb 2. Pain. 2022. PMID: 35121697 Free PMC article.
-
Estrogen Receptor-A in Medial Preoptic Area Contributes to Sex Difference of Mice in Response to Sevoflurane Anesthesia.Neurosci Bull. 2022 Jul;38(7):703-719. doi: 10.1007/s12264-022-00825-w. Epub 2022 Feb 17. Neurosci Bull. 2022. PMID: 35175557 Free PMC article.
References
-
- Barton M. (2012) Position paper: The membrane estrogen receptor GPER—Clues and questions. Steroids 77:935–942 - PubMed
-
- Bereiter DA, Cioffi JL, Bereiter DF. (2005) Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol 50:971–979 - PubMed
-
- Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. (2007a) Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 321:839–847 - PubMed
-
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, et al. (2006) Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

