Transesophageal Echocardiography in Healthy Young Adult Male Baboons (Papio hamadryas anubis): Normal Cardiac Anatomy and Function in Subhuman Primates Compared to Humans
- PMID: 24707162
- PMCID: PMC3974204
- DOI: 10.1016/j.ppedcard.2013.09.002
Transesophageal Echocardiography in Healthy Young Adult Male Baboons (Papio hamadryas anubis): Normal Cardiac Anatomy and Function in Subhuman Primates Compared to Humans
Abstract
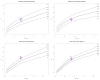
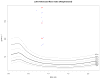
Implantable, viable tissue engineered cardiovascular constructs are rapidly approaching clinical translation. Species typically utilized as preclinical large animal models are food stock ungulates for which cross species biological and genomic differences with humans are great. Multiple authorities have recommended developing subhuman primate models for testing regenerative surgical strategies to mitigate xenotransplant inflammation. However, there is a lack of specific quantitative cardiac imaging comparisons between humans and the genomically similar baboons (Papio hamadryas anubis). This study was undertaken to translate to baboons transesophageal echocardiographic functional and dimensional criteria defined as necessary for defining cardiac anatomy and function in the perioperative setting. Seventeen young, healthy baboons (approximately 30 kg, similar to 5 year old children) were studied to determine whether the requisite 11 views and 52 measurement parameters could be reliably acquired by transesophageal echocardiography (TEE). The obtained measurements were compared to human adult normative literature values and to a large relational database of pediatric "normal heart" echo measurements. Comparisons to humans, when normalized to BSA, revealed a trend in baboons toward larger mitral and aortic valve effective orifice areas and much larger left ventricular muscle mass and wall thickness, but similar pulmonary and tricuspid valves. By modifying probe positioning relative to human techniques, all recommended TEE views except transgastric could be replicated. To supplement, two transthoracic apical views were discovered that in baboons could reliably replace the transgastric TEE view. Thus, all requisite echo views could be obtained for a complete cardiac evaluation in Papio hamadryas anubis to noninvasively quantify cardiac structural anatomy, physiology, and dimensions. Despite similarities between the species, there are subtle and important physiologic and anatomic differences when compared to human.
Keywords: Animal; Disease Models; Echocardiography; Heart; Heart Hypertrophy; Heart Valve Prosthesis; Papio; Primate; Transesophageal.
Figures





References
-
- Barnhart GR, Jones M, Ishihara T, Chavez AM, Rose DM, Ferrans VJ. Bioprosthetic valvular failure. Clinical and pathological observations in an experimental animal model. J Thorac Cardiovasc Surg. 1982;83:618–31. - PubMed
-
- Gallegos RP, Nockel PJ, Rivard AL, Bianco RW. The current state of in-vivo pre-clinical animal models for heart valve evaluation. J Heart Valve Dis. 2005;14:423–32. - PubMed
-
- Grehan JF, Casagrande I, Oliveira EL, et al. A juvenile sheep model for the long-term evaluation of stentless bioprostheses implanted as aortic root replacements. J Heart Valve Dis. 2001;10:505–12. - PubMed
-
- Salerno CT, Droel J, Bianco RW. Current state of in vivo preclinical heart valve evaluation. J Heart Valve Dis. 1998;7:158–62. - PubMed
-
- Neethling W, Hodge A, Glancy R. Kangaroo versus Freestyle® stentless bioprostheses in a juvenile sheep model: hemodynamic performance and calcification behavior. J Card Surg. 2005;20:29–34. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
