Reduction in cardiovascular risk using a proactive multifactorial intervention is consistent among patients residing in Pacific Asian and non-Pacific Asian regions: a CRUCIAL trial subanalysis
- PMID: 24707184
- PMCID: PMC3971943
- DOI: 10.2147/VHRM.S54586
Reduction in cardiovascular risk using a proactive multifactorial intervention is consistent among patients residing in Pacific Asian and non-Pacific Asian regions: a CRUCIAL trial subanalysis
Abstract
Background: Few trials have compared different approaches to cardiovascular disease prevention among Pacific Asian (PA) populations. The Cluster Randomized Usual Care versus Caduet Investigation Assessing Long-term-risk (CRUCIAL) trial demonstrated that a proactive multifactorial intervention (PMI) approach (based on single-pill amlodipine/atorvastatin) resulted in a greater reduction in calculated Framingham 10-year coronary heart disease (CHD) risk compared with usual care (UC) among hypertensive patients with additional risk factors. One-third of CRUCIAL patients resided in the PA region. The aim of this subanalysis was to compare two approaches to cardiovascular risk factor management (PMI versus UC) among patients residing in PA and non-PA regions.
Methods: This subanalysis of the CRUCIAL trial compared treatment-related changes in calculated CHD risk among patients residing in PA and non-PA regions. Sensitivity analyses were conducted among men and women and those with and without diabetes.
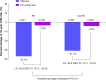
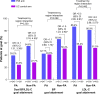
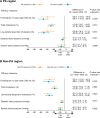
Results: Overall, 448 patients (31.6%) resided in the PA region and 969 patients (68.4%) resided in non-PA regions. The PMI approach was more effective in reducing calculated CHD risk versus UC in both PA (-37.1% versus -3.5%; P<0.001) and non-PA regions (-31.1% versus -4.2%; P<0.001); region interaction P=0.131. PA patients had slightly greater reductions in total cholesterol compared with non-PA patients. PA patients without diabetes had slightly greater reductions in CHD risk compared with non-PA patients. Treatment effects were similar in men and women and those with diabetes.
Conclusion: The PMI approach was more effective in reducing calculated Framingham 10-year CHD risk compared with UC among men and women with and without diabetes residing in the PA and non-PA region.
Keywords: anticholesteremic agents; antihypertensive agents; cardiovascular disease; clinical trial; hypertension; risk factors.
Figures


References
-
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A, FEVER Study Group The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23(12):2157–2172. - PubMed
-
- JATOS Study Group Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens Res. 2008;31(12):2115–2127. - PubMed
-
- Muramatsu T, Matsushita K, Yamashita K, et al. NAGOYA HEART Study Investigators Comparison between valsartan and amlodipine regarding cardiovascular morbidity and mortality in hypertensive patients with glucose intolerance: NAGOYA HEART Study. Hypertension. 2012;59(3):580–586. - PubMed
-
- Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. - PubMed
-
- Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104(23):2855–2864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

