Antigenic and mechanistic characterization of anti-AMPA receptor encephalitis
- PMID: 24707504
- PMCID: PMC3972064
- DOI: 10.1002/acn3.43
Antigenic and mechanistic characterization of anti-AMPA receptor encephalitis
Abstract
Objective: Anti-AMPAR encephalitis is a recently discovered disorder characterized by the presence of antibodies in serum or cerebrospinal fluid against the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor. Here, we examine the antigenic specificity of anti-AMPAR antibodies, screen for new patients, and evaluate functional effects of antibody treatment of neurons.
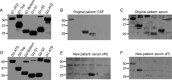
Methods: We developed a fusion protein-based western blotting test for anti-AMPAR encephalitis antibodies. Antibody specificity was also evaluated using immunocytochemistry of HEK293 cells expressing deletion mutants of AMPAR subunits. Purified patient IgG or AMPAR antibody-depleted IgG was applied to live neuronal cultures; amplitude and frequency of miniature excitatory postsynaptic currents (mEPSCs) were measured to evaluate functional effects of antibodies.
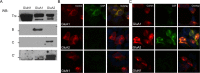
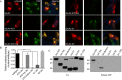
Results: Using both immunocytochemistry and fusion protein western blots, we defined an antigenic region of the receptor in the bottom lobe of the amino terminal domain. Additionally, we used fusion proteins to screen 70 individuals with neurologic symptoms of unknown cause and 44 patients with no neurologic symptoms or symptoms of known neuroimmunological origin for anti-AMPAR antibodies. Fifteen of the 70 individuals had anti-AMPAR antibodies, with broader antigenic reactivity patterns. Using purified IgG from an individual of the original cohort of anti-AMPAR encephalitis patients and a newly discovered patient, we found that application of IgG from either patient cohort caused an AMPAR antibody-dependent decrease in the amplitude and frequency of mEPSCs in cultured neurons.
Interpretation: These results indicate that anti-AMPAR antibodies are widespread and functionally relevant; given the robust response of patients to immunomodulation, this represents a significant treatable patient population.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

