2012 Division of medicinal chemistry award address. Trekking the cannabinoid road: a personal perspective
- PMID: 24707904
- PMCID: PMC4064474
- DOI: 10.1021/jm500220s
2012 Division of medicinal chemistry award address. Trekking the cannabinoid road: a personal perspective
Abstract
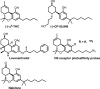
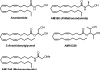
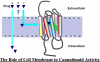
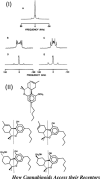
My involvement with the field of cannabinoids spans close to 3 decades and covers a major part of my scientific career. It also reflects the robust progress in this initially largely unexplored area of biology. During this period of time, I have witnessed the growth of modern cannabinoid biology, starting from the discovery of its two receptors and followed by the characterization of its endogenous ligands and the identification of the enzyme systems involved in their biosynthesis and biotransformation. I was fortunate enough to start at the beginning of this new era and participate in a number of the new discoveries. It has been a very exciting journey. With coverage of some key aspects of my work during this period of "modern cannabinoid research," this Award Address, in part historical, intends to give an account of how the field grew, the key discoveries, and the most promising directions for the future.
Figures










Similar articles
-
Medicinal chemistry of cannabinoids.Clin Pharmacol Ther. 2015 Jun;97(6):553-8. doi: 10.1002/cpt.115. Epub 2015 Apr 16. Clin Pharmacol Ther. 2015. PMID: 25801236 Free PMC article. Review.
-
Targeting the cannabinoid system for pain relief?Acta Anaesthesiol Taiwan. 2013 Dec;51(4):161-70. doi: 10.1016/j.aat.2013.10.004. Epub 2013 Dec 25. Acta Anaesthesiol Taiwan. 2013. PMID: 24529672 Review.
-
FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels.Curr Opin Investig Drugs. 2010 Jan;11(1):51-62. Curr Opin Investig Drugs. 2010. PMID: 20047159 Review.
-
Pharmacological actions of cannabinoids.Handb Exp Pharmacol. 2005;(168):1-51. doi: 10.1007/3-540-26573-2_1. Handb Exp Pharmacol. 2005. PMID: 16596770 Review.
-
Cannabinoid 1 (CB1) receptor--pharmacology, role in pain and recent developments in emerging CB1 agonists.CNS Neurol Disord Drug Targets. 2011 Aug;10(5):536-44. doi: 10.2174/187152711796235005. CNS Neurol Disord Drug Targets. 2011. PMID: 21631407 Review.
Cited by
-
Keys to Lipid Selection in Fatty Acid Amide Hydrolase Catalysis: Structural Flexibility, Gating Residues and Multiple Binding Pockets.PLoS Comput Biol. 2015 Jun 25;11(6):e1004231. doi: 10.1371/journal.pcbi.1004231. eCollection 2015 Jun. PLoS Comput Biol. 2015. PMID: 26111155 Free PMC article.
-
Synthesis of Functionalized Cannabilactones.Molecules. 2020 Feb 6;25(3):684. doi: 10.3390/molecules25030684. Molecules. 2020. PMID: 32041131 Free PMC article.
-
Novel C-Ring-Hydroxy-Substituted Controlled Deactivation Cannabinergic Analogues.J Med Chem. 2016 Jul 28;59(14):6903-19. doi: 10.1021/acs.jmedchem.6b00717. Epub 2016 Jul 13. J Med Chem. 2016. PMID: 27367336 Free PMC article.
-
Quantification of ∆9-tetrahydrocannabinol, 11-OH-THC, THC-COOH, hexahydrocannabinol, and cannabidiol in human plasma and blood by liquid chromatography-tandem mass spectrometry.J Anal Toxicol. 2025 Feb 15;49(2):85-95. doi: 10.1093/jat/bkae094. J Anal Toxicol. 2025. PMID: 39656878 Free PMC article.
-
Cannabinoid-2 Agonism with AM2301 Mitigates Morphine-Induced Respiratory Depression.Cannabis Cannabinoid Res. 2021 Oct;6(5):401-412. doi: 10.1089/can.2020.0076. Epub 2020 Nov 13. Cannabis Cannabinoid Res. 2021. PMID: 33998869 Free PMC article.
References
-
- Charalambous A, Yan G, Houston DB, Howlett AC, Compton DR, Martin BR, Makriyannis A. 5′-Azido-delta 8-THC: a novel photoaffinity label for the cannabinoid receptor. J Med Chem. 1992;35:3076–3079. - PubMed
-
- Burstein SH, Audette CA, Charalambous A, Doyle SA, Guo Y, Hunter SA, Makriyannis A. Detection of cannabinoid receptors by photoaffinity labelling. Biochem Biophys Res Commun. 1991;176:492–497. - PubMed
-
- Pertwee R, Griffin G, Fernando S, Li X, Hill A, Makriyannis A. AM630, a competitive cannabinoid receptor antagonist. Life Sci. 1995;56:1949–1955. - PubMed
-
- Picone RP, Khanolkar AD, Xu W, Ayotte LA, Thakur GA, Hurst DP, Abood ME, Reggio PH, Fournier DJ, Makriyannis A. (−)-7′-Isothiocyanato-11-hydroxy-1′,1′-dimethylheptylhexahydrocannabinol (AM841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Mol Pharmacol. 2005;68:1623–1635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

