Angiotensin II-induced protein kinase D activates the ATF/CREB family of transcription factors and promotes StAR mRNA expression
- PMID: 24708239
- PMCID: PMC4060184
- DOI: 10.1210/en.2013-1485
Angiotensin II-induced protein kinase D activates the ATF/CREB family of transcription factors and promotes StAR mRNA expression
Abstract
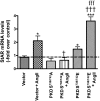
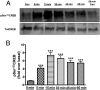
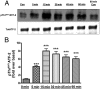
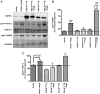
Aldosterone synthesis is initiated upon the transport of cholesterol from the outer to the inner mitochondrial membrane, where the cholesterol is hydrolyzed to pregnenolone. This process is the rate-limiting step in acute aldosterone production and is mediated by the steroidogenic acute regulatory (StAR) protein. We have previously shown that angiotensin II (AngII) activation of the serine/threonine protein kinase D (PKD) promotes acute aldosterone production in bovine adrenal glomerulosa cells, but the mechanism remains unclear. Thus, the purpose of this study was to determine the downstream signaling effectors of AngII-stimulated PKD activity. Our results demonstrate that overexpression of the constitutively active serine-to-glutamate PKD mutant enhances, whereas the dominant-negative serine-to-alanine PKD mutant inhibits, AngII-induced StAR mRNA expression relative to the vector control. PKD has been shown to phosphorylate members of the activating transcription factor (ATF)/cAMP response element binding protein (CREB) family of leucine zipper transcription factors, which have been shown previously to bind the StAR proximal promoter and induce StAR mRNA expression. In primary glomerulosa cells, AngII induces ATF-2 and CREB phosphorylation in a time-dependent manner. Furthermore, overexpression of the constitutively active PKD mutant enhances the AngII-elicited phosphorylation of ATF-2 and CREB, and the dominant-negative mutant inhibits this response. Furthermore, the constitutively active PKD mutant increases the binding of phosphorylated CREB to the StAR promoter. Thus, these data provide insight into the previously reported role of PKD in AngII-induced acute aldosterone production, providing a mechanism by which PKD may be mediating steroidogenesis in primary bovine adrenal glomerulosa cells.
Figures


References
-
- Müller J. Aldosterone: the minority hormone of the adrenal cortex. Steroids. 1995;60(1):2–9 - PubMed
-
- Quinn SJ, Williams GH. Regulation of aldosterone secretion. Annu Rev Physiol. 1988;50:409–426 - PubMed
-
- Calhoun DA. Aldosterone and cardiovascular disease: smoke and fire. Circulation. 2006;114:2572–2574 - PubMed
-
- Delcayre C, Swynghedauw B. Molecular mechanisms of myocardial remodeling. The role of aldosterone. J Mol Cell Cardiol. 2002;34(12):1577–1584 - PubMed
-
- Rocha R, Stier CT, Jr, Kifor I, et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141(10):3871–3878 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

