Gene expression profile analysis of Manila clam (Ruditapes philippinarum) hemocytes after a Vibrio alginolyticus challenge using an immune-enriched oligo-microarray
- PMID: 24708293
- PMCID: PMC4234419
- DOI: 10.1186/1471-2164-15-267
Gene expression profile analysis of Manila clam (Ruditapes philippinarum) hemocytes after a Vibrio alginolyticus challenge using an immune-enriched oligo-microarray
Abstract
Background: The Manila clam (Ruditapes philippinarum) is a cultured bivalve with worldwide commercial importance, and diseases cause high economic losses. For this reason, interest in the immune genes in this species has recently increased. The present work describes the construction of the first R. philippinarum microarray containing immune-related hemocyte sequences and its application to study the gene transcription profiles of hemocytes from clams infected with V. alginolyticus through a time course.
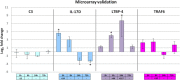
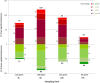
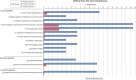
Results: The complete set of sequences from R. philippinarum available in the public databases and the hemocyte sequences enriched in immune transcripts were assembled successfully. A total of 12,156 annotated sequences were used to construct the 8 × 15 k oligo-microarray. The microarray experiments yielded a total of 579 differentially expressed transcripts. Using the gene expression results, the associated Gene Ontology terms and the enrichment analysis, we found different response mechanisms throughout the experiment. Genes related to signaling, transcription and apoptosis, such as IL-17D, NF-κB or calmodulin, were typically expressed as early as 3 hours post-challenge (hpc), while characteristic immune genes, such as PGRPs, FREPs and defense proteins appeared later at 8 hpc. This immune-triggering response could have affected a high number of processes that seemed to be activated 24 hpc to overcome the Vibrio challenge, including the expression of many cytoskeleton molecules, which is indicative of the active movement of hemocytes. In fact functional studies showed an increment in apoptosis, necrosis or cell migration after the infection. Finally, 72 hpc, activity returned to normal levels, and more than 50% of the genes were downregulated in a negative feedback of all of the previously active processes.
Conclusions: Using a new version of the R. philippinarum oligo-microarray, a putative timing for the response against a Vibrio infection was established. The key point to overcome the challenge seemed to be 8 hours after the challenge, when we detected immune functions that could lead to the destruction of the pathogen and the activation of a wide variety of processes related to homeostasis and defense. These results highlight the importance of a fast response in bivalves and the effectiveness of their innate immune system.
Figures



References
-
- Gestal C, Roch P, Renault T, Pallavicini A, Paillard C, Novoa B, Oubella R, Venier P, Figueras A. Study of diseases and the immune system of bivalves using molecular biology and genomics. Rev Fish Sci. 2008;16:131–154.
-
- Paillard C, Leroux F, Borrego JJ. Bacterial disease in marine bivalves, Review of recent studies. Trends and evolution. Aquat Living Resour. 2004;17:477–498. doi: 10.1051/alr:2004054. - DOI
-
- Villalba A, Reece KS, Ordás MC, Casas SM, Figueras A. Perkinsosis in molluscs: A review. Aquat Living Resour. 2004;17:411–432. doi: 10.1051/alr:2004050. - DOI
Publication types
MeSH terms
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

