Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study
- PMID: 24708472
- PMCID: PMC3994204
- DOI: 10.1186/1465-9921-15-39
Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study
Abstract
Background: Recent studies have demonstrated that mesenchymal stem cells (MSCs) modulate the immune response and reduce lung injury in animal models. Currently, no clinical studies of the effects of MSCs in acute respiratory distress syndrome (ARDS) exist. The objectives of this study were first to examine the possible adverse events after systemic administration of allogeneic adipose-derived MSCs in ARDS patients and second to determine potential efficacy of MSCs on ARDS.
Methods: Twelve adult patients meeting the Berlin definition of acute respiratory distress syndrome with a PaO2/FiO2 ratio of < 200 were randomized to receive allogeneic adipose-derived MSCs or placebo in a 1:1 fashion. Patients received one intravenous dose of 1 × 106 cells/kg of body weight or saline. Possible side effects were monitored after treatment. Acute lung injury biomarkers, including IL-6, IL-8 and surfactant protein D (SP-D), were examined to determine the effects of MSCs on lung injury and inflammation.
Results: There were no infusion toxicities or serious adverse events related to MSCs administration and there were no significant differences in the overall number of adverse events between the two groups. Length of hospital stay, ventilator-free days and ICU-free days at day 28 after treatment were similar. There were no changes in biomarkers examined in the placebo group. In the MSCs group, serum SP-D levels at day 5 were significantly lower than those at day 0 (p = 0.027) while the changes in IL-8 levels were not significant. The IL-6 levels at day 5 showed a trend towards lower levels as compared with day 0, but this trend was not statistically significant (p = 0.06).
Conclusions: Administration of allogeneic adipose-derived MSCs appears to be safe and feasible in the treatment of ARDS. However, the clinical effect with the doses of MSCs used is weak, and further optimization of this strategy will probably be required to reach the goal of reduced alveolar epithelial injury in ARDS.
Trial registration: Clinical trials.gov, NCT01902082.
Figures

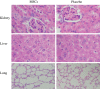
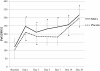
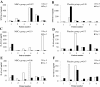
References
-
- Bernardo ME, Ball LM, Cometa AM, Roelofs H, Zecca M, Avanzini MA, Bertaina A, Vinti L, Lankester A, Maccario R, Ringden O, Le Blanc K, Egeler RM, Fibbe WE, Locatelli F. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2011;46:200–207. doi: 10.1038/bmt.2010.87. - DOI - PubMed
-
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

