Allelic variation in the canine Cox-2 promoter causes hypermethylation of the canine Cox-2 promoter in clinical cases of renal dysplasia
- PMID: 24708682
- PMCID: PMC4234983
- DOI: 10.1186/1868-7083-6-7
Allelic variation in the canine Cox-2 promoter causes hypermethylation of the canine Cox-2 promoter in clinical cases of renal dysplasia
Abstract
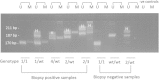
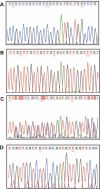
Background: Novel allelic variants in the promoter of the canine cyclooxygenase-2 (Cox-2) gene are associated with renal dysplasia (RD). These variants consist of either deletions of putative SP1 transcription factor-binding sites or insertions of tandem repeats of SP1-binding sites located in the CpG island just upstream of the ATG translation initiation site. The canine Cox-2 gene was studied because Cox-2-deficient mice have renal abnormalities and a pathology that is strikingly similar to RD in dogs.
Findings: The allelic variants were associated with hypermethylation of the Cox-2 promoter only in clinical cases of RD. The wild-type allele was never methylated, even in clinical cases that were heterozygous for a mutant allele. In cases that were biopsy-negative, the promoter remained unmethylated, regardless of the genotype. Methylated DNA was found in DNA from various adult tissues of dogs with clinical RD.
Conclusions: The mechanism of action of the allelic variation in the canine Cox-2 promoter most likely involves variation in the extent of epigenetic downregulation of this gene. This epigenetic downregulation must have occurred early in development because methylated Cox-2 promoter DNA sequences are found in various adult tissues.
Figures

References
-
- Dressler GR. Epigenetics, development, and the kidney. J Am Soc Nephrol. 2008;11:2060–2070. - PubMed
-
- Okamoto T, Hino O. Expression of cyclooxygenase-1 and -2 mRNA in rat tissues: tissue-specific difference in the expression of the basal level of mRNA. Int J Mol Med. 2000;4:455–457. - PubMed
-
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee D, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;23:406–409. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

