Epidermal growth factor receptor inhibitor ameliorates excessive astrogliosis and improves the regeneration microenvironment and functional recovery in adult rats following spinal cord injury
- PMID: 24708754
- PMCID: PMC4030311
- DOI: 10.1186/1742-2094-11-71
Epidermal growth factor receptor inhibitor ameliorates excessive astrogliosis and improves the regeneration microenvironment and functional recovery in adult rats following spinal cord injury
Abstract
Background: Astrogliosis is a common phenomenon after spinal cord injury (SCI). Although this process exerts positive effects on axonal regeneration, excessive astrogliosis imparts negative effects on neuronal repair and recovery. Epidermal growth factor receptor (EGFR) pathway is critical to the regulation of reactive astrogliosis, and therefore is a potential target of therapeutics to better control the response. In this report, we aim to investigate whether blocking EGFR signaling using an EGFR tyrosine kinase specific inhibitor can attenuate reactive astrogliosis and promote functional recovery after a traumatic SCI.
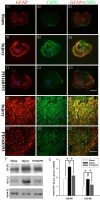
Method: The astrocyte scratch injury model in vitro and the weight-drop SCI model in vivo were used as model systems. PD168393 was used to inhibit EGFR signaling activation. Astrocytic activation and phosphorylated EGFR (pEGFR) were observed after immunofluorescence staining and Western blot analysis. The rate of proliferation was determined by immunofluorescence detection of BrdU-incorporating cells located next to the wound. The levels of TNF-α, iNOS, COX-2 and IL-1β in the culture medium under different conditions were assayed by ELISA. Western blot was performed to semi-quantify the expression of EGFR/pEGFR, glial fibrillary acid protein (GFAP) and chondroitin sulfate proteoglycans (CSPGs). Myelin was stained by Luxol Fast Blue Staining. Cresyl violet eosin staining was performed to analyze the lesion cavity volume and neuronal survival following injury. Finally, functional scoring and residual urine recording were performed to show the rats' recovery.
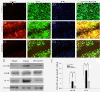
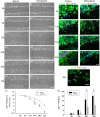
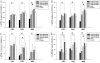
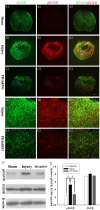
Results: EGFR phosphorylation was found to parallel astrocyte activation, and EGFR inhibitor PD168393 potently inhibited scratch-induced reactive astrogliosis and proinflammatory cytokine/mediator secretion of reactive astrocytes in vitro. Moreover, local administration of PD168393 in the injured area suppressed CSPGs production and glial scar formation, and resulted in reduced demyelination and neuronal loss, which correlated with remarkable hindlimb motor function and bladder improvement in SCI rats.
Conclusions: The specific EGFR inhibitor PD168393 can ameliorate excessive reactive astrogliosis and facilitate a more favorable environment for axonal regeneration after SCI. As such, EGFR inhibitor may be a promising therapeutic intervention in CNS injury.
Figures
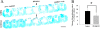

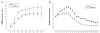
Similar articles
-
Inhibiting epidermal growth factor receptor attenuates reactive astrogliosis and improves functional outcome after spinal cord injury in rats.Neurochem Int. 2011 Jun;58(7):812-9. doi: 10.1016/j.neuint.2011.03.007. Epub 2011 Mar 21. Neurochem Int. 2011. PMID: 21402118
-
Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury.J Neuroinflammation. 2012 Jul 23;9:178. doi: 10.1186/1742-2094-9-178. J Neuroinflammation. 2012. PMID: 22824323 Free PMC article.
-
Triptolide promotes spinal cord repair by inhibiting astrogliosis and inflammation.Glia. 2010 Jun;58(8):901-15. doi: 10.1002/glia.20972. Glia. 2010. PMID: 20155820
-
Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects.Mol Neurobiol. 2012 Oct;46(2):251-64. doi: 10.1007/s12035-012-8287-4. Epub 2012 Jun 9. Mol Neurobiol. 2012. PMID: 22684804 Review.
-
Astrocyte reactivity and astrogliosis after spinal cord injury.Neurosci Res. 2018 Jan;126:39-43. doi: 10.1016/j.neures.2017.10.004. Epub 2017 Oct 17. Neurosci Res. 2018. PMID: 29054466 Review.
Cited by
-
Glial Scar-a Promising Target for Improving Outcomes After CNS Injury.J Mol Neurosci. 2020 Mar;70(3):340-352. doi: 10.1007/s12031-019-01417-6. Epub 2019 Nov 27. J Mol Neurosci. 2020. PMID: 31776856 Review.
-
Repurposed anti-cancer epidermal growth factor receptor inhibitors: mechanisms of neuroprotective effects in Alzheimer's disease.Neural Regen Res. 2022 Sep;17(9):1913-1918. doi: 10.4103/1673-5374.332132. Neural Regen Res. 2022. PMID: 35142667 Free PMC article. Review.
-
Mesenchymal Stem Cells in the Treatment of Human Spinal Cord Injury: The Effect on Individual Values of pNF-H, GFAP, S100 Proteins and Selected Growth Factors, Cytokines and Chemokines.Curr Issues Mol Biol. 2022 Jan 24;44(2):578-596. doi: 10.3390/cimb44020040. Curr Issues Mol Biol. 2022. PMID: 35723326 Free PMC article.
-
A Novel Network Pharmacology Strategy Based on the Universal Effectiveness-Common Mechanism of Medical Herbs Uncovers Therapeutic Targets in Traumatic Brain Injury.Drug Des Devel Ther. 2024 Apr 16;18:1175-1188. doi: 10.2147/DDDT.S450895. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38645986 Free PMC article.
-
Microglial dyshomeostasis drives perineuronal net and synaptic loss in a CSF1R+/- mouse model of ALSP, which can be rescued via CSF1R inhibitors.Sci Adv. 2021 Aug 25;7(35):eabg1601. doi: 10.1126/sciadv.abg1601. Print 2021 Aug. Sci Adv. 2021. PMID: 34433559 Free PMC article.
References
-
- Usher LC, Johnstone A, Erturk A, Hu Y, Strikis D, Wanner IB, Moorman S, Lee JW, Min J, Ha HH, Duan Y, Hoffman S, Goldberg JL, Bradke F, Chang YT, Lemmon VP, Bixby JL. A chemical screen identifies novel compounds that overcome glial-mediated inhibition of neuronal regeneration. J Neurosci. 2010;30:4693–4706. doi: 10.1523/JNEUROSCI.0302-10.2010. - DOI - PMC - PubMed
-
- Norsted Gregory E, Delaney A, Abdelmoaty S, Bas DB, Codeluppi S, Wigerblad G, Svensson CI. Pentoxifylline and propentofylline prevent proliferation and activation of the mammalian target of rapamycin and mitogen activated protein kinase in cultured spinal astrocytes. J Neurosci Res. 2013;91:300–312. doi: 10.1002/jnr.23144. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

