Randomised phase II study of docetaxel plus vandetanib versus docetaxel followed by vandetanib in patients with persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: SWOG S0904
- PMID: 24709487
- PMCID: PMC4098779
- DOI: 10.1016/j.ejca.2014.03.005
Randomised phase II study of docetaxel plus vandetanib versus docetaxel followed by vandetanib in patients with persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: SWOG S0904
Abstract
Background: Vandetanib is an oral tyrosine kinase inhibitor of VEGFR-2/3, EGFR and RET, which has demonstrated clinical activity as a single agent and in combination with taxanes. We explored the efficacy, safety and toxicity of docetaxel and vandetanib in women with recurrent ovarian cancer (OC).
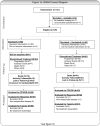
Methods: Women with refractory or progressive OC were randomised 1:1 to docetaxel (75 mg/m(2), IV)+vandetanib (100mg daily, PO, D+V) or docetaxel (75 mg/m(2), D). Up to three additional cytotoxic regimens for recurrence and prior anti-angiogenic agents (as primary therapy) were allowed. The primary end-point was progression free survival (PFS). The study had 84% power to detect a PFS hazard ratio of 0.65, using a one-sided P of 0.1. This corresponds to an increase in median PFS from 3.6 months to 5.6 months. Patients progressing on D were allowed to receive single agent vandetanib (D → V).
Results: 131 Patients were enrolled; two were excluded. 16% had received prior anti-angiogenic therapy. The median PFS estimates were 3.0 mos (D+V) versus 3.5 (D); HR: 0.99 (80% CI: 0.79-1.26). 61 Patients on D+V were assessable for toxicity; 20(33%) had treatment-related Grade (G) 4 events, primarily haematologic. Similarly, 17 (27%) of 64 patients receiving D had G4 events, primarily haematologic. 27 Evaluable patients crossed-over to V. 1/27(4%) experienced a G4 event. G3 diarrhoea was observed in 4% D → V patients. Median OS was 14 mos (D+V) versus 18 mos (D → V); HR(OS): 1.25 (80% CI: 0.93-1.68). Crossover vandetanib response was 4% (1/27 evaluable patients). High plasma IL-8 levels were associated with response to D+V.
Conclusions: Combination docetaxel+vandetanib did not prolong PFS relative to docetaxel alone in OC patients. No unexpected safety issues were identified.
Keywords: Chemotherapy; Clinical trial; Epithelial cancer; Fallopian tube cancer; Ovarian cancer; Primary peritoneal cancer; Randomised phase II; Taxanes; Vandetanib.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. - PubMed
-
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35. discussion 35-6. - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine. 1995;1:27–31. - PubMed
-
- Carmeliet P. Developmental biology. One cell, two fates. Nature. 2000;408:43, 45. - PubMed
-
- Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angiomagenesis? Nat Med. 2000;6:1102–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA37981/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- CA16385/CA/NCI NIH HHS/United States
- U10 CA045461/CA/NCI NIH HHS/United States
- U10 CA105409/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- CA58723/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA016385/CA/NCI NIH HHS/United States
- CA35128/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- U10 CA035128/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- CA45808/CA/NCI NIH HHS/United States
- U10 CA035421/CA/NCI NIH HHS/United States
- U10 CA180846/CA/NCI NIH HHS/United States
- CA58882/CA/NCI NIH HHS/United States
- CA35421/CA/NCI NIH HHS/United States
- CA76132/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- CA105409/CA/NCI NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- CA45461/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- UG1 CA233340/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- CA42777/CA/NCI NIH HHS/United States
- UG1 CA189808/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UL1 TR000371/TR/NCATS NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

