Induction of Cell Death Mechanisms and Apoptosis by Nanosecond Pulsed Electric Fields (nsPEFs)
- PMID: 24709649
- PMCID: PMC3972658
- DOI: 10.3390/cells2010136
Induction of Cell Death Mechanisms and Apoptosis by Nanosecond Pulsed Electric Fields (nsPEFs)
Abstract
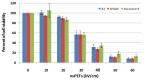
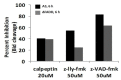
Pulse power technology using nanosecond pulsed electric fields (nsPEFs) offers a new stimulus to modulate cell functions or induce cell death for cancer cell ablation. New data and a literature review demonstrate fundamental and basic cellular mechanisms when nsPEFs interact with cellular targets. NsPEFs supra-electroporate cells creating large numbers of nanopores in all cell membranes. While nsPEFs have multiple cellular targets, these studies show that nsPEF-induced dissipation of ΔΨm closely parallels deterioration in cell viability. Increases in intracellular Ca2+ alone were not sufficient for cell death; however, cell death depended of the presence of Ca2+. When both events occur, cell death ensues. Further, direct evidence supports the hypothesis that pulse rise-fall times or high frequency components of nsPEFs are important for decreasing ΔΨm and cell viability. Evidence indicates in Jurkat cells that cytochrome c release from mitochondria is caspase-independent indicating an absence of extrinsic apoptosis and that cell death can be caspase-dependent and -independent. The Ca2+ dependence of nsPEF-induced dissipation of ΔΨm suggests that nanoporation of inner mitochondria membranes is less likely and effects on a Ca2+-dependent protein(s) or the membrane in which it is embedded are more likely a target for nsPEF-induced cell death. The mitochondria permeability transition pore (mPTP) complex is a likely candidate. Data demonstrate that nsPEFs can bypass cancer mutations that evade apoptosis through mechanisms at either the DISC or the apoptosome.
Figures








References
-
- Sersa G., Cemazar M., Miklavcic D. Antitumor effectiveness of electrochemotherapy with cis-diamminedichloroplatinum(II) in mice. Cancer Res. 1995;55:3450–3455. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

