Regulation of Apoptosis by Inhibitors of Apoptosis (IAPs)
- PMID: 24709650
- PMCID: PMC3972657
- DOI: 10.3390/cells2010163
Regulation of Apoptosis by Inhibitors of Apoptosis (IAPs)
Abstract
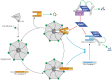
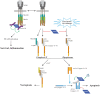
Inhibitors of Apoptosis (IAPs) are a family of proteins with various biological functions including regulation of innate immunity and inflammation, cell proliferation, cell migration and apoptosis. They are characterized by the presence of at least one N-terminal baculoviral IAP repeat (BIR) domain involved in protein-protein interaction. Most of them also contain a C-terminal RING domain conferring an E3-ubiquitin ligase activity. In drosophila, IAPs are essential to ensure cell survival, preventing the uncontrolled activation of the apoptotic protease caspases. In mammals, IAPs can also regulate apoptosis through controlling caspase activity and caspase-activating platform formation. Mammalian IAPs, mainly X-linked IAP (XIAP) and cellular IAPs (cIAPs) appeared to be important determinants of the response of cells to endogenous or exogenous cellular injuries, able to convert the survival signal into a cell death-inducing signal. This review highlights the role of IAP in regulating apoptosis in Drosophila and Mammals.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

