Pericytes, mesenchymal stem cells and the wound healing process
- PMID: 24709801
- PMCID: PMC3972668
- DOI: 10.3390/cells2030621
Pericytes, mesenchymal stem cells and the wound healing process
Abstract
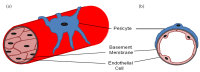
Pericytes are cells that reside on the wall of the blood vessels and their primary function is to maintain the vessel integrity. Recently, it has been realized that pericytes have a much greater role than just the maintenance of vessel integrity essential for the development and formation of a vascular network. Pericytes also have stem cell-like properties and are seemingly able to differentiate into adipocytes, chondrocytes, osteoblasts and granulocytes, leading them to be identified as mesenchymal stem cells (MSCs). More recently it has been suggested that pericytes play a key role in wound healing, whereas the beneficial effects of MSCs in accelerating the wound healing response has been recognized for some time. In this review, we collate the most recent data on pericytes, particularly their role in vessel formation and how they can affect the wound healing process.
Figures
References
-
- Hirschi K.K., D’Amore P.A. Pericytes in the microvasculature. Cardiovasc. Res. 1996;32:687–698. - PubMed
-
- Etchevers H.C., Vincent C., le Douarin N.M., Couly G.F. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources