LaSSO, a strategy for genome-wide mapping of intronic lariats and branch points using RNA-seq
- PMID: 24709818
- PMCID: PMC4079972
- DOI: 10.1101/gr.166819.113
LaSSO, a strategy for genome-wide mapping of intronic lariats and branch points using RNA-seq
Abstract
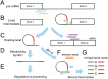
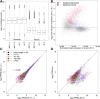
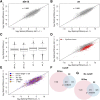
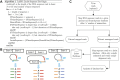
Both canonical and alternative splicing of RNAs are governed by intronic sequence elements and produce transient lariat structures fastened by branch points within introns. To map precisely the location of branch points on a genomic scale, we developed LaSSO (Lariat Sequence Site Origin), a data-driven algorithm which utilizes RNA-seq data. Using fission yeast cells lacking the debranching enzyme Dbr1, LaSSO not only accurately identified canonical splicing events, but also pinpointed novel, but rare, exon-skipping events, which may reflect aberrantly spliced transcripts. Compromised intron turnover perturbed gene regulation at multiple levels, including splicing and protein translation. Notably, Dbr1 function was also critical for the expression of mitochondrial genes and for the processing of self-spliced mitochondrial introns. LaSSO showed better sensitivity and accuracy than algorithms used for computational branch-point prediction or for empirical branch-point determination. Even when applied to a human data set acquired in the presence of debranching activity, LaSSO identified both canonical and exon-skipping branch points. LaSSO thus provides an effective approach for defining high-resolution maps of branch-site sequences and intronic elements on a genomic scale. LaSSO should be useful to validate introns and uncover branch-point sequences in any eukaryote, and it could be integrated into RNA-seq pipelines.
© 2014 Bitton et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Cheng Z, Menees TM 2011. RNA splicing and debranching viewed through analysis of RNA lariats. Mol Genet Genomics 286: 395–410 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
