Systems biology as an integrated platform for bioinformatics, systems synthetic biology, and systems metabolic engineering
- PMID: 24709875
- PMCID: PMC3972654
- DOI: 10.3390/cells2040635
Systems biology as an integrated platform for bioinformatics, systems synthetic biology, and systems metabolic engineering
Abstract
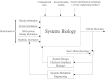
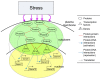
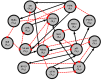
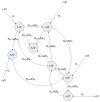
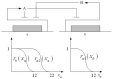
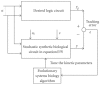
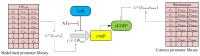
Systems biology aims at achieving a system-level understanding of living organisms and applying this knowledge to various fields such as synthetic biology, metabolic engineering, and medicine. System-level understanding of living organisms can be derived from insight into: (i) system structure and the mechanism of biological networks such as gene regulation, protein interactions, signaling, and metabolic pathways; (ii) system dynamics of biological networks, which provides an understanding of stability, robustness, and transduction ability through system identification, and through system analysis methods; (iii) system control methods at different levels of biological networks, which provide an understanding of systematic mechanisms to robustly control system states, minimize malfunctions, and provide potential therapeutic targets in disease treatment; (iv) systematic design methods for the modification and construction of biological networks with desired behaviors, which provide system design principles and system simulations for synthetic biology designs and systems metabolic engineering. This review describes current developments in systems biology, systems synthetic biology, and systems metabolic engineering for engineering and biology researchers. We also discuss challenges and future prospects for systems biology and the concept of systems biology as an integrated platform for bioinformatics, systems synthetic biology, and systems metabolic engineering.
Figures



Similar articles
-
Plant and bacterial systems biology as platform for plant synthetic bio(techno)logy.J Biotechnol. 2012 Jul 31;160(1-2):80-90. doi: 10.1016/j.jbiotec.2012.01.014. Epub 2012 Jan 25. J Biotechnol. 2012. PMID: 22306308 Review.
-
Modular design: Implementing proven engineering principles in biotechnology.Biotechnol Adv. 2019 Nov 15;37(7):107403. doi: 10.1016/j.biotechadv.2019.06.002. Epub 2019 Jun 7. Biotechnol Adv. 2019. PMID: 31181317 Review.
-
A new approach to an old problem: synthetic biology tools for human disease and metabolism.Cold Spring Harb Symp Quant Biol. 2011;76:145-54. doi: 10.1101/sqb.2011.76.010686. Epub 2011 Dec 14. Cold Spring Harb Symp Quant Biol. 2011. PMID: 22169233 Review.
-
Cell-free synthetic biology: Engineering in an open world.Synth Syst Biotechnol. 2017 Mar 3;2(1):23-27. doi: 10.1016/j.synbio.2017.02.003. eCollection 2017 Mar. Synth Syst Biotechnol. 2017. PMID: 29062958 Free PMC article. Review.
-
Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts.Biotechnol Adv. 2021 Mar-Apr;47:107695. doi: 10.1016/j.biotechadv.2021.107695. Epub 2021 Jan 16. Biotechnol Adv. 2021. PMID: 33465474 Review.
Cited by
-
Developing a gene expression classifier for breast cancer diagnosis.Med Biol Eng Comput. 2025 Mar 13. doi: 10.1007/s11517-025-03329-7. Online ahead of print. Med Biol Eng Comput. 2025. PMID: 40080330
-
Key Immune Events of the Pathomechanisms of Early Cardioembolic Stroke: Multi-Database Mining and Systems Biology Approach.Int J Mol Sci. 2016 Feb 27;17(3):305. doi: 10.3390/ijms17030305. Int J Mol Sci. 2016. PMID: 26927091 Free PMC article.
-
Systems Approach to Pathogenic Mechanism of Type 2 Diabetes and Drug Discovery Design Based on Deep Learning and Drug Design Specifications.Int J Mol Sci. 2020 Dec 26;22(1):166. doi: 10.3390/ijms22010166. Int J Mol Sci. 2020. PMID: 33375269 Free PMC article.
-
Crosslink: An R Package for Network Visualization of Grouped Nodes.Front Genet. 2021 Jul 16;12:706854. doi: 10.3389/fgene.2021.706854. eCollection 2021. Front Genet. 2021. PMID: 34335702 Free PMC article.
-
Drug Target Identification and Drug Repurposing in Psoriasis through Systems Biology Approach, DNN-Based DTI Model and Genome-Wide Microarray Data.Int J Mol Sci. 2023 Jun 12;24(12):10033. doi: 10.3390/ijms241210033. Int J Mol Sci. 2023. PMID: 37373186 Free PMC article.
References
-
- Peri S., Navarro J.D., Amanchy R., Kristiansen T.Z., Jonnalagadda C.K., Surendranath V., Niranjan V., Muthusamy B., Gandhi T.K.B., Gronborg M., et al. Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 2003;13:2363–2371. doi: 10.1101/gr.1680803. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

