WRN loss induces switching of telomerase-independent mechanisms of telomere elongation
- PMID: 24709898
- PMCID: PMC3977986
- DOI: 10.1371/journal.pone.0093991
WRN loss induces switching of telomerase-independent mechanisms of telomere elongation
Abstract
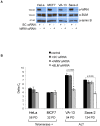
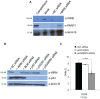
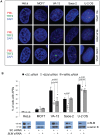
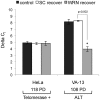
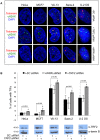
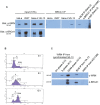
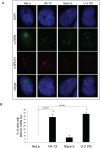
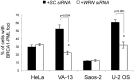
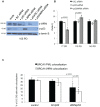
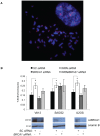
Telomere maintenance can occur in the presence of telomerase or in its absence, termed alternative lengthening of telomeres (ALT). ALT adds telomere repeats using recombination-based processes and DNA repair proteins that function in homologous recombination. Our previous work reported that the RecQ-like BLM helicase is required for ALT and that it unwinds telomeric substrates in vitro. WRN is also a RecQ-like helicase that shares many biochemical functions with BLM. WRN interacts with BLM, unwinds telomeric substrates, and co-localizes to ALT-associated PML bodies (APBs), suggesting that it may also be required for ALT processes. Using long-term siRNA knockdown of WRN in three ALT cell lines, we show that some, but not all, cell lines require WRN for telomere maintenance. VA-13 cells require WRN to prevent telomere loss and for the formation of APBs; Saos-2 cells do not. A third ALT cell line, U-2 OS, requires WRN for APB formation, however WRN loss results in p53-mediated apoptosis. In the absence of WRN and p53, U-2 OS cells undergo telomere loss for an intermediate number of population doublings (50-70), at which point they maintain telomere length even with the continued loss of WRN. WRN and the tumor suppressor BRCA1 co-localize to APBs in VA-13 and U-2 OS, but not in Saos-2 cells. WRN loss in U-2 OS is associated with a loss of BRCA1 from APBs. While the loss of WRN significantly increases telomere sister chromatid exchanges (T-SCE) in these three ALT cell lines, loss of both BRCA1 and WRN does not significantly alter T-SCE. This work demonstrates that ALT cell lines use different telomerase-independent maintenance mechanisms that variably require the WRN helicase and that some cells can switch from one mechanism to another that permits telomere elongation in the absence of WRN. Our data suggest that BRCA1 localization may define these mechanisms.
Conflict of interest statement
Figures
Similar articles
-
Association of BLM and BRCA1 during Telomere Maintenance in ALT Cells.PLoS One. 2014 Aug 1;9(8):e103819. doi: 10.1371/journal.pone.0103819. eCollection 2014. PLoS One. 2014. PMID: 25084169 Free PMC article.
-
The roles of WRN and BLM RecQ helicases in the Alternative Lengthening of Telomeres.Nucleic Acids Res. 2012 Nov;40(21):10809-20. doi: 10.1093/nar/gks862. Epub 2012 Sep 18. Nucleic Acids Res. 2012. PMID: 22989712 Free PMC article.
-
Telomere length heterogeneity in ALT cells is maintained by PML-dependent localization of the BTR complex to telomeres.Genes Dev. 2020 May 1;34(9-10):650-662. doi: 10.1101/gad.333963.119. Epub 2020 Mar 26. Genes Dev. 2020. PMID: 32217664 Free PMC article.
-
Telomere recombination and alternative telomere lengthening mechanisms.Front Biosci (Landmark Ed). 2013 Jan 1;18(1):1-20. doi: 10.2741/4084. Front Biosci (Landmark Ed). 2013. PMID: 23276906 Review.
-
Unwinding protein complexes in ALTernative telomere maintenance.J Cell Biochem. 2010 Jan 1;109(1):7-15. doi: 10.1002/jcb.22388. J Cell Biochem. 2010. PMID: 19911388 Free PMC article. Review.
Cited by
-
Telomerase Regulation from Beginning to the End.Genes (Basel). 2016 Sep 14;7(9):64. doi: 10.3390/genes7090064. Genes (Basel). 2016. PMID: 27649246 Free PMC article. Review.
-
The Molecular Mechanisms and Therapeutic Prospects of Alternative Lengthening of Telomeres (ALT).Cancers (Basel). 2023 Mar 23;15(7):1945. doi: 10.3390/cancers15071945. Cancers (Basel). 2023. PMID: 37046606 Free PMC article. Review.
-
Locking the gates of immortality: targeting alternative lengthening of telomeres (ALT) pathways.Med Oncol. 2025 Feb 18;42(3):78. doi: 10.1007/s12032-025-02627-2. Med Oncol. 2025. PMID: 39964637 Review.
-
DNA Replication Origins and Fork Progression at Mammalian Telomeres.Genes (Basel). 2017 Mar 28;8(4):112. doi: 10.3390/genes8040112. Genes (Basel). 2017. PMID: 28350373 Free PMC article. Review.
-
TelNet - a database for human and yeast genes involved in telomere maintenance.BMC Genet. 2018 May 18;19(1):32. doi: 10.1186/s12863-018-0617-8. BMC Genet. 2018. PMID: 29776332 Free PMC article.
References
-
- Lundblad V, Blackburn EH (1993) An alternative pathway for yeast telomere maintenance rescues est1− senescence. Cell 73: 347–360. - PubMed
-
- Fasching CL, Bower K, Reddel RR (2005) Telomerase-independent telomere length maintenance in the absence of alternative lengthening of telomeres-associated promyelocytic leukemia bodies. Cancer Res 65: 2722–2729. - PubMed
-
- Marciniak RA, Cavazos D, Montellano R, Chen Q, Guarente L, et al. (2005) A novel telomere structure in a human alternative lengthening of telomere cell line. Cancer Res 65: 2730–2737. - PubMed
-
- Dunham MA, Neumann AA, Fasching CL, Reddel RR (2000) Telomere maintenance by recombination in human cells. Nat Genet 26: 447–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

