Dopamine signaling in C. elegans is mediated in part by HLH-17-dependent regulation of extracellular dopamine levels
- PMID: 24709946
- PMCID: PMC4065251
- DOI: 10.1534/g3.114.010819
Dopamine signaling in C. elegans is mediated in part by HLH-17-dependent regulation of extracellular dopamine levels
Abstract
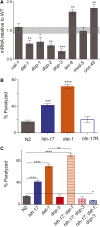
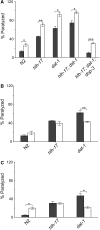
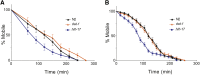
In Caenorhabditis elegans, the dopamine transporter DAT-1 regulates synaptic dopamine (DA) signaling by controlling extracellular DA levels. In dat-1(ok157) animals, DA is not taken back up presynaptically but instead reaches extrasynpatic sites, where it activates the dopamine receptor DOP-3 on choligeneric motor neurons and causes animals to become paralyzed in water. This phenotype is called swimming-induced paralysis (SWIP) and is dependent on dat-1 and dop-3. Upstream regulators of dat-1 and dop-3 have yet to be described in C. elegans. In our previous studies, we defined a role for HLH-17 during dopamine response through its regulation of the dopamine receptors. Here we continue our characterization of the effects of HLH-17 on dopamine signaling. Our results suggest that HLH-17 acts downstream of dopamine synthesis to regulate the expression of dop-3 and dat-1. First, we show that hlh-17 animals display a SWIP phenotype that is consistent with its regulation of dop-3 and dat-1. Second, we show that this behavior is enhanced by treatment with the dopamine reuptake inhibitor, bupropion, in both hlh-17 and dat-1 animals, a result suggesting that SWIP behavior is regulated via a mechanism that is both dependent on and independent of DAT-1. Third, and finally, we show that although the SWIP phenotype of hlh-17 animals is unresponsive to the dopamine agonist, reserpine, and to the antidepressant, fluoxetine, hlh-17 animals are not defective in acetylcholine signaling. Taken together, our work suggests that HLH-17 is required to maintain normal levels of dopamine in the synaptic cleft through its regulation of dop-3 and dat-1.
Keywords: acetylcholine signaling; bupropion; dopamine receptor; fluoxetine; reserpine.
Copyright © 2014 Felton and Johnson.
Figures

References
-
- Biedermann B., Frohlich E., Grosche J., Wagner H. J., Reichenbach A., 1995. Mammalian Muller (glial) cells express functional D2 dopamine receptors. Neuroreport 6: 609–612 - PubMed
-
- Bolanos C. A., Trksak G. H., Cohen O. S., Jackson D., 2002. Differential serotonergic inhibition of in vitro striatal [3H]acetylcholine release in prenatally cocaine-exposed male and female rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 26: 1339–1348 - PubMed
-
- Borodovsky N., Ponomaryov T., Frenkel S., Levkowitz G., 2009. Neural protein Olig2 acts upstream of the transcriptional regulator Sim1 to specify diencephalic dopaminergic neurons. Dev. Dyn. 238: 826–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
