Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth
- PMID: 24710033
- PMCID: PMC4162516
- DOI: 10.1161/CIRCULATIONAHA.114.009124
Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth
Abstract
Background: Bronchopulmonary dysplasia and emphysema are life-threatening diseases resulting from impaired alveolar development or alveolar destruction. Both conditions lack effective therapies. Angiogenic growth factors promote alveolar growth and contribute to alveolar maintenance. Endothelial colony-forming cells (ECFCs) represent a subset of circulating and resident endothelial cells capable of self-renewal and de novo vessel formation. We hypothesized that resident ECFCs exist in the developing lung, that they are impaired during arrested alveolar growth in experimental bronchopulmonary dysplasia, and that exogenous ECFCs restore disrupted alveolar growth.
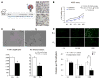
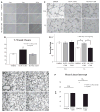
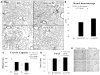
Methods and results: Human fetal and neonatal rat lungs contain ECFCs with robust proliferative potential, secondary colony formation on replating, and de novo blood vessel formation in vivo when transplanted into immunodeficient mice. In contrast, human fetal lung ECFCs exposed to hyperoxia in vitro and neonatal rat ECFCs isolated from hyperoxic alveolar growth-arrested rat lungs mimicking bronchopulmonary dysplasia proliferated less, showed decreased clonogenic capacity, and formed fewer capillary-like networks. Intrajugular administration of human cord blood-derived ECFCs after established arrested alveolar growth restored lung function, alveolar and lung vascular growth, and attenuated pulmonary hypertension. Lung ECFC colony- and capillary-like network-forming capabilities were also restored. Low ECFC engraftment and the protective effect of cell-free ECFC-derived conditioned media suggest a paracrine effect. Long-term (10 months) assessment of ECFC therapy showed no adverse effects with persistent improvement in lung structure, exercise capacity, and pulmonary hypertension.
Conclusions: Impaired ECFC function may contribute to arrested alveolar growth. Cord blood-derived ECFC therapy may offer new therapeutic options for lung diseases characterized by alveolar damage.
Keywords: angiogenesis inducing agents; hypertension, pulmonary; lung diseases; stem cells.
© 2014 American Heart Association, Inc.
Figures






Comment in
-
Expanding the pool of stem cell therapy for lung growth and repair.Circulation. 2014 May 27;129(21):2091-3. doi: 10.1161/CIRCULATIONAHA.114.009727. Epub 2014 Apr 7. Circulation. 2014. PMID: 24710032 Free PMC article. No abstract available.
References
-
- Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. 2013;25:329–337. - PubMed
-
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. - PubMed
-
- Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32:321–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

