Oxygen in the regulation of intestinal epithelial transport
- PMID: 24710059
- PMCID: PMC4080932
- DOI: 10.1113/jphysiol.2013.270249
Oxygen in the regulation of intestinal epithelial transport
Abstract
The transport of fluid, nutrients and electrolytes to and from the intestinal lumen is a primary function of epithelial cells. Normally, the intestine absorbs approximately 9 l of fluid and 1 kg of nutrients daily, driven by epithelial transport processes that consume large amounts of cellular energy and O2. The epithelium exists at the interface of the richly vascularised mucosa, and the anoxic luminal environment and this steep O2 gradient play a key role in determining the expression pattern of proteins involved in fluid, nutrient and electrolyte transport. However, the dynamic nature of the splanchnic circulation necessitates that the epithelium can evoke co-ordinated responses to fluctuations in O2 availability, which occur either as a part of the normal digestive process or as a consequence of several pathophysiological conditions. While it is known that hypoxia-responsive signals, such as reactive oxygen species, AMP-activated kinase, hypoxia-inducible factors, and prolyl hydroxylases are all important in regulating epithelial responses to altered O2 supply, our understanding of the molecular mechanisms involved is still limited. Here, we aim to review the current literature regarding the role that O2 plays in regulating intestinal transport processes and to highlight areas of research that still need to be addressed.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures
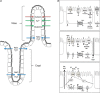
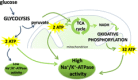
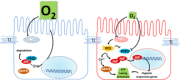
Similar articles
-
The Fast Lane of Hypoxic Adaptation: Glucose Transport Is Modulated via A HIF-Hydroxylase-AMPK-Axis in Jejunum Epithelium.Int J Mol Sci. 2019 Oct 9;20(20):4993. doi: 10.3390/ijms20204993. Int J Mol Sci. 2019. PMID: 31601024 Free PMC article.
-
Hypoxia and gastrointestinal disease.J Mol Med (Berl). 2007 Dec;85(12):1295-300. doi: 10.1007/s00109-007-0277-z. Epub 2007 Nov 20. J Mol Med (Berl). 2007. PMID: 18026919 Review.
-
The response of five intestinal cell lines to anoxic conditions in vitro.Biol Cell. 2019 Sep;111(9):232-244. doi: 10.1111/boc.201800076. Epub 2019 Jun 25. Biol Cell. 2019. PMID: 31187884
-
Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia.Am J Physiol Cell Physiol. 2015 Sep 15;309(6):C350-60. doi: 10.1152/ajpcell.00191.2015. Epub 2015 Jul 15. Am J Physiol Cell Physiol. 2015. PMID: 26179603 Free PMC article. Review.
-
Glucose transport across lagomorph jejunum epithelium is modulated by AMP-activated protein kinase under hypoxia.J Appl Physiol (1985). 2017 Dec 1;123(6):1487-1500. doi: 10.1152/japplphysiol.00436.2017. Epub 2017 Aug 31. J Appl Physiol (1985). 2017. PMID: 28860168
Cited by
-
The Fast Lane of Hypoxic Adaptation: Glucose Transport Is Modulated via A HIF-Hydroxylase-AMPK-Axis in Jejunum Epithelium.Int J Mol Sci. 2019 Oct 9;20(20):4993. doi: 10.3390/ijms20204993. Int J Mol Sci. 2019. PMID: 31601024 Free PMC article.
-
Making in vitro conditions more reflective of in vivo conditions for research on the teleost gastrointestinal tract.J Exp Biol. 2024 Oct 1;227(19):jeb246440. doi: 10.1242/jeb.246440. Epub 2024 Oct 11. J Exp Biol. 2024. PMID: 39392112 Free PMC article. Review.
-
Formula feeding and systemic hypoxia synergistically induce intestinal hypoxia in experimental necrotizing enterocolitis.Pediatr Surg Int. 2016 Dec;32(12):1115-1119. doi: 10.1007/s00383-016-3997-8. Epub 2016 Nov 4. Pediatr Surg Int. 2016. PMID: 27815640
-
Evolutionary Adaptations of Parasitic Flatworms to Different Oxygen Tensions.Antioxidants (Basel). 2022 May 31;11(6):1102. doi: 10.3390/antiox11061102. Antioxidants (Basel). 2022. PMID: 35739999 Free PMC article. Review.
-
Intestinal Inflammation as a Dysbiosis of Energy Procurement: New Insights into an Old Topic.Gut Microbes. 2021 Jan-Dec;13(1):1-20. doi: 10.1080/19490976.2021.1880241. Gut Microbes. 2021. PMID: 33583319 Free PMC article. Review.
References
-
- Alhan E, Usta A, Cekic A, Saglam K, Turkyilmaz S, Cinel A. A study on 107 patients with acute mesenteric ischemia over 30 years. Int J Surg. 2012;10:510–513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

