Transient impairment of the axolemma following regional anaesthesia by lidocaine in humans
- PMID: 24710060
- PMCID: PMC4221817
- DOI: 10.1113/jphysiol.2014.270827
Transient impairment of the axolemma following regional anaesthesia by lidocaine in humans
Abstract
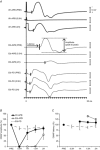
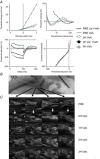
The local anaesthetic lidocaine is known to block voltage-gated Na(+) channels (VGSCs), although at high concentration it was also reported to block other ion channel currents as well as to alter lipid membranes. The aim of this study was to investigate whether the clinical regional anaesthetic action of lidocaine could be accounted for solely by the block of VGSCs or whether other mechanisms are also relevant. We tested the recovery of motor axon conduction and multiple measures of excitability by 'threshold-tracking' after ultrasound-guided distal median nerve regional anaesthesia in 13 healthy volunteers. Lidocaine caused rapid complete motor axon conduction block localized at the wrist. Within 3 h, the force of the abductor pollicis brevis muscle and median motor nerve conduction studies returned to normal. In contrast, the excitability of the motor axons at the wrist remained markedly impaired as indicated by a 7-fold shift of the stimulus-response curves to higher currents with partial recovery by 6 h and full recovery by 24 h. The strength-duration properties were abnormal with markedly increased rheobase and reduced strength-duration time constant. The changes in threshold during electrotonus, especially during depolarization, were markedly reduced. The recovery cycle showed increased refractoriness and reduced superexcitability. The excitability changes were only partly similar to those previously observed after poisoning with the VGSC blocker tetrodotoxin. Assuming an unaltered ion-channel gating, modelling indicated that, apart from up to a 4-fold reduction in the number of functioning VGSCs, lidocaine also caused a decrease of passive membrane resistance and an increase of capacitance. Our data suggest that the lidocaine effects, even at clinical 'sub-blocking' concentrations, could reflect, at least in part, a reversible structural impairment of the axolemma.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures




Similar articles
-
The effects of mexiletine on excitability properties of human median motor axons.Clin Neurophysiol. 2005 Feb;116(2):284-9. doi: 10.1016/j.clinph.2004.08.014. Clin Neurophysiol. 2005. PMID: 15661106 Clinical Trial.
-
Na(v)1.8 channelopathy in mutant mice deficient for myelin protein zero is detrimental to motor axons.Brain. 2011 Feb;134(Pt 2):585-601. doi: 10.1093/brain/awq336. Epub 2010 Dec 17. Brain. 2011. PMID: 21169333
-
Evidence for axonal membrane hyperpolarization in multifocal motor neuropathy with conduction block.Brain. 2002 Mar;125(Pt 3):664-75. doi: 10.1093/brain/awf041. Brain. 2002. PMID: 11872621
-
[Lidocaine in oncological surgery: the role of blocking in voltage-gated sodium channels. A narrative review].Braz J Anesthesiol. 2020 Sep-Oct;70(5):527-533. doi: 10.1016/j.bjan.2020.04.018. Epub 2020 Sep 3. Braz J Anesthesiol. 2020. PMID: 32951865 Free PMC article. Review.
-
Blocking channels to metastasis: targeting sodium transport in breast cancer.Breast Cancer Res. 2023 Nov 10;25(1):140. doi: 10.1186/s13058-023-01741-1. Breast Cancer Res. 2023. PMID: 37950273 Free PMC article. Review.
Cited by
-
Relief of Secondary Headaches with High Thoracic Erector Spinae Plane Block.Local Reg Anesth. 2020 Jun 22;13:49-55. doi: 10.2147/LRA.S249250. eCollection 2020. Local Reg Anesth. 2020. PMID: 32606918 Free PMC article.
-
An in Vivo Mouse Model to Investigate the Effect of Local Anesthetic Nanomedicines on Axonal Conduction and Excitability.Front Neurosci. 2018 Jul 26;12:494. doi: 10.3389/fnins.2018.00494. eCollection 2018. Front Neurosci. 2018. PMID: 30093852 Free PMC article.
-
Effects of Mexiletine and Lacosamide on Nerve Excitability in Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study.Clin Pharmacol Ther. 2022 Nov;112(5):1008-1019. doi: 10.1002/cpt.2694. Epub 2022 Jul 19. Clin Pharmacol Ther. 2022. PMID: 35762293 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

