Computational design of binding proteins to EGFR domain II
- PMID: 24710267
- PMCID: PMC3977815
- DOI: 10.1371/journal.pone.0092513
Computational design of binding proteins to EGFR domain II
Abstract
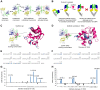
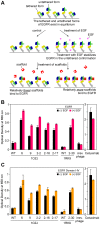
We developed a process to produce novel interactions between two previously unrelated proteins. This process selects protein scaffolds and designs protein interfaces that bind to a surface patch of interest on a target protein. Scaffolds with shapes complementary to the target surface patch were screened using an exhaustive computational search of the human proteome and optimized by directed evolution using phage display. This method was applied to successfully design scaffolds that bind to epidermal growth factor receptor (EGFR) domain II, the interface of EGFR dimerization, with high reactivity toward the target surface patch of EGFR domain II. One potential application of these tailor-made protein interactions is the development of therapeutic agents against specific protein targets.
Conflict of interest statement
Figures




References
-
- Yamada T, Bork P (2009) Evolution of biomolecular networks: lessons from metabolic and protein interactions. Nat Rev Mol Cell Biol 10: 791–803. - PubMed
-
- Levy ED, Pereira-Leal JB (2008) Evolution and dynamics of protein interactions and networks. Curr Opin Struct Biol 18: 349–357. - PubMed
-
- Kim PM, Lu LJ, Xia Y, Gerstein MB (2006) Relating three-dimensional structures to protein networks provides evolutionary insights. Science 314: 1938–1941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

