Microglia trigger astrocyte-mediated neuroprotection via purinergic gliotransmission
- PMID: 24710318
- PMCID: PMC3948352
- DOI: 10.1038/srep04329
Microglia trigger astrocyte-mediated neuroprotection via purinergic gliotransmission
Abstract
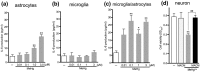
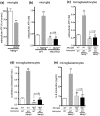
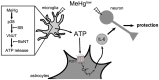
Microglia are highly sensitive to even small changes in the brain environment, such as invasion of non-hazardous toxicants or the presymptomatic state of diseases. However, the physiological or pathophysiological consequences of their responses remain unknown. Here, we report that cultured microglia sense low concentrations of the neurotoxicant methylmercury (MeHg(low)) and provide neuroprotection against MeHg, for which astrocytes are also required. When exposed to MeHg(low), microglia exocytosed ATP via p38 MAPK- and vesicular nucleotide transporter (VNUT)-dependent mechanisms. Astrocytes responded to the microglia-derived ATP via P2Y1 receptors and released interleukin-6 (IL-6), thereby protecting neurons against MeHg(low). These neuroprotective actions were also observed in organotypic hippocampal slices from wild-type mice, but not in slices prepared from VNUT knockout or P2Y1 receptor knockout mice. These findings suggest that microglia sense and respond to even non-hazardous toxicants such as MeHg(low) and change their phenotype into a neuroprotective one, for which astrocytic support is required.
Figures




Similar articles
-
Astrocytes protect neurons against methylmercury via ATP/P2Y(1) receptor-mediated pathways in astrocytes.PLoS One. 2013;8(2):e57898. doi: 10.1371/journal.pone.0057898. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23469098 Free PMC article.
-
Transformation of Astrocytes to a Neuroprotective Phenotype by Microglia via P2Y1 Receptor Downregulation.Cell Rep. 2017 May 9;19(6):1151-1164. doi: 10.1016/j.celrep.2017.04.047. Cell Rep. 2017. PMID: 28494865
-
Anti-Depressant Fluoxetine Reveals its Therapeutic Effect Via Astrocytes.EBioMedicine. 2018 Jun;32:72-83. doi: 10.1016/j.ebiom.2018.05.036. Epub 2018 Jun 8. EBioMedicine. 2018. PMID: 29887330 Free PMC article.
-
Vesicular nucleotide transporter (VNUT): appearance of an actress on the stage of purinergic signaling.Purinergic Signal. 2017 Sep;13(3):387-404. doi: 10.1007/s11302-017-9568-1. Epub 2017 Jun 14. Purinergic Signal. 2017. PMID: 28616712 Free PMC article. Review.
-
Physiopathological roles of vesicular nucleotide transporter (VNUT), an essential component for vesicular ATP release.Biochim Biophys Acta Biomembr. 2020 Dec 1;1862(12):183408. doi: 10.1016/j.bbamem.2020.183408. Epub 2020 Jul 9. Biochim Biophys Acta Biomembr. 2020. PMID: 32652056 Review.
Cited by
-
Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity.Mol Psychiatry. 2017 May;22(5):760-773. doi: 10.1038/mp.2016.130. Epub 2016 Aug 16. Mol Psychiatry. 2017. PMID: 27528462 Free PMC article.
-
Stem cell factor induces polarization of microglia to the neuroprotective phenotype in vitro.Heliyon. 2018 Oct 1;4(10):e00837. doi: 10.1016/j.heliyon.2018.e00837. eCollection 2018 Oct. Heliyon. 2018. PMID: 30294687 Free PMC article.
-
Glial Contribution to Excitatory and Inhibitory Synapse Loss in Neurodegeneration.Front Cell Neurosci. 2019 Feb 26;13:63. doi: 10.3389/fncel.2019.00063. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30863284 Free PMC article. Review.
-
Astrocyte Circadian Timekeeping in Brain Health and Neurodegeneration.Adv Exp Med Biol. 2021;1344:87-110. doi: 10.1007/978-3-030-81147-1_6. Adv Exp Med Biol. 2021. PMID: 34773228
-
Human central nervous system astrocytes support survival and activation of B cells: implications for MS pathogenesis.J Neuroinflammation. 2018 Apr 19;15(1):114. doi: 10.1186/s12974-018-1136-2. J Neuroinflammation. 2018. PMID: 29673365 Free PMC article.
References
-
- Nimmerjahn A., Kirchhoff F. & Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). - PubMed
-
- Davalos D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758 (2005). - PubMed
-
- Ouchi Y. et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol 57, 168–175 (2005). - PubMed
-
- Kingwell K. Neurodegenerative disease: Microglia in early disease stages. Nat Rev Neurol 8, 475 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

