Can ELISPOT Be Applied to A Clinical Setting as A Diagnostic Utility for Neuroborreliosis?
- PMID: 24710421
- PMCID: PMC3901091
- DOI: 10.3390/cells1020153
Can ELISPOT Be Applied to A Clinical Setting as A Diagnostic Utility for Neuroborreliosis?
Abstract
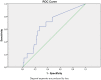
The aim of this prospective study was to investigate the diagnostic performance of Borrelia (Bb)-induced interferon (IFN)-γ secretion detected by ELISPOT modified to be feasible for clinical laboratories as a supplementary test to the laboratory diagnosis of Lyme neuroborreliosis (LNB) in an endemic setting. Between 2002 and 2004, patients with symptoms of suspected clinical LNB were included in a study conducted on the Åland islands in the Finnish archipelago, which is a hyper-endemic area for Lyme borreliosis (LB). Fourteen patients with confirmed LNB and 103 patients with non-LNB were included, and the numbers of spontaneous and Bb-induced IFN-γ-secreting cells were assayed by the ELISPOT test. The ELISPOT assay showed a weak diagnostic performance with a sensitivity of 36% and a specificity of 82%. The findings in this study show that this ELISPOT-assay modified to be feasible in clinical routine laboratories is not useful as a supplementary diagnostic tool in the laboratory diagnosis of patients with clinically suspected LNB.
Figures

References
-
- Stanek G., Fingerle V., Hunfeld K.P., Jaulhac B., Kaiser R., Krause A., Kristoferitsch W., O’Connell S., Ornstein K., Strle F., et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in europe. Clin. Microbiol. Infect. 2011;17:697–699. doi: 10.1111/j.1469-0691.2010.03307.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

