Neuroantigen-Specific CD4 Cells Expressing Interferon-γ (IFN-γ), Interleukin (IL)-2 and IL-3 in a Mutually Exclusive Manner Prevail in Experimental Allergic Encephalomyelitis (EAE)
- PMID: 24710491
- PMCID: PMC3901106
- DOI: 10.3390/cells1030576
Neuroantigen-Specific CD4 Cells Expressing Interferon-γ (IFN-γ), Interleukin (IL)-2 and IL-3 in a Mutually Exclusive Manner Prevail in Experimental Allergic Encephalomyelitis (EAE)
Abstract
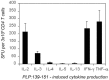
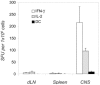
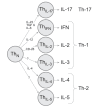
Experimental allergic encephalomyelitis (EAE) is mediated by neuroantigen-specific pro-inflammatory T cells of the Th1 and Th17 effector class. Th-17 cells can be clearly defined by expression of IL-17, but not IFN-γ, IL-2 or IL-3. Th1 cells do not express IL-17, but it is unclear presently to what extent they co-express the cytokines canonically assigned to Th1 immunity (i.e., IFN-γ, IL-2 and IL-3) and whether CD4 cells producing these cytokines indeed belong to a single Th1 lineage. It is also unclear to what extent the Th1 response in EAE entails polyfunctional T cells that co-express IFN-γ and IL-2. Therefore, we dissected the Th1 cytokine signature of neuroantigen-specific CD4 cells studying at single cell resolution co-expression of IFN-γ, IL-2 and IL-3 using dual color cytokine ELISPOT analysis. Shortly after immunization, in the draining lymph nodes (dLN), the overall cytokine signature of the neuroantigen-specific CD4 cells was highly type 1-polarized, but IFN-γ, IL-2, and IL-3 were each secreted by different CD4 cells in a mutually exclusive manner. This single cell - single cytokine profile was stable through the course of chronic EAE-polyfunctional CD4 cells co-expressing IL-2 and IFN-γ presented less than 5% of the neuroantigen-specific T cells, even in the inflamed CNS itself. The neuroantigen-specific CD4 cells that expressed IFN-γ, IL-2 and IL-3 in a mutually exclusive manner exhibited similar functional avidities and kinetics of cytokine production, but showed different tissue distributions. These data suggest that Th1 cells do not belong to a single lineage, but different Th1 subpopulations jointly mediate Th1 immunity.
Figures



References
-
- Saparov A., Elson C., Devore-Carter D., Bucy R., Weaver C. Single-cell analyses of CD4+ T cells from alpha beta T cell receptor- transgenic mice: A distinct mucosal cytokine phenotype in the absence of transgene-specific antigen. Eur. J. Immunol. 1997;27:1774–1781. doi: 10.1002/eji.1830270727. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

