Harnessing photochemical internalization with dual degradable nanoparticles for combinatorial photo-chemotherapy
- PMID: 24710504
- PMCID: PMC3988806
- DOI: 10.1038/ncomms4623
Harnessing photochemical internalization with dual degradable nanoparticles for combinatorial photo-chemotherapy
Abstract
Light-controlled drug delivery systems constitute an appealing means to direct and confine drug release spatiotemporally at the site of interest with high specificity. However, the utilization of light-activatable systems is hampered by the lack of suitable drug carriers that respond sharply to visible light stimuli at clinically relevant wavelengths. Here, a new class of self-assembling, photo- and pH-degradable polymers of the polyacetal family is reported, which is combined with photochemical internalization to control the intracellular trafficking and release of anticancer compounds. The polymers are synthesized by simple and scalable chemistries and exhibit remarkably low photolysis rates at tunable wavelengths over a large range of the spectrum up to the visible and near infrared regime. The combinational pH and light mediated degradation facilitates increased therapeutic potency and specificity against model cancer cell lines in vitro. Increased cell death is achieved by the synergistic activity of nanoparticle-loaded anticancer compounds and reactive oxygen species accumulation in the cytosol by simultaneous activation of porphyrin molecules and particle photolysis.
Figures
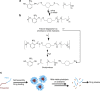

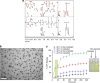
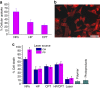

References
-
- Fang J., Nakamura H. & Maeda H. The EPR effect: unique features of tumour blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 63, 136–151 (2011). - PubMed
-
- Schroeder A. et al. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 12, 39–50 (2012). - PubMed
-
- Danhier F., Feron O. & Preat V. To exploit the tumour microenvironment: passive and active tumour targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 148, 135–146 (2010). - PubMed
-
- Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2, 347–360 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

