Novel role of the serine protease inhibitor elafin in gluten-related disorders
- PMID: 24710505
- PMCID: PMC4219532
- DOI: 10.1038/ajg.2014.48
Novel role of the serine protease inhibitor elafin in gluten-related disorders
Abstract
Objectives: Elafin, an endogenous serine protease inhibitor, modulates colonic inflammation. We investigated the role of elafin in celiac disease (CD) using human small intestinal tissues and in vitro assays of gliadin deamidation. We also investigated the potential beneficial effects of elafin in a mouse model of gluten sensitivity.
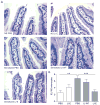
Methods: Epithelial elafin expression in the small intestine of patients with active CD, treated CD, and controls without CD was determined by immunofluorescence. Interaction of elafin with human tissue transglutaminase-2 (TG-2) was investigated in vitro. The 33-mer peptide, a highly immunogenic gliadin peptide, was incubated with TG-2 and elafin at different concentrations. The degree of deamidation of the 33-mer peptide was analyzed by liquid chromatography-mass spectrometry. Elafin was delivered to the intestine of gluten-sensitive mice using a recombinant Lactococcus lactis vector. Small intestinal barrier function, inflammation, proteolytic activity, and zonula occludens-1 (ZO-1) expression were assessed.
Results: Elafin expression in the small intestinal epithelium was lower in patients with active CD compared with control patients. In vitro, elafin significantly slowed the kinetics of the deamidation of the 33-mer peptide to its more immunogenic form. Treatment of gluten-sensitive mice with elafin delivered by the L. lactis vector normalized inflammation, improved permeability, and maintained ZO-1 expression.
Conclusions: The decreased elafin expression in the small intestine of patients with active CD, the reduction of 33-mer peptide deamidation by elafin, coupled to the barrier enhancing and anti-inflammatory effects observed in gluten-sensitive mice, suggest that this molecule may have pathophysiological and therapeutic importance in gluten-related disorders.
Figures




Comment in
-
Use of elafin in celiac disease.Turk J Gastroenterol. 2015 Jan;26(1):93-4. doi: 10.5152/tjg.2015.0115. Turk J Gastroenterol. 2015. PMID: 25698289 No abstract available.
References
-
- Verdu EF. Editorial: Can Gluten Contribute to Irritable Bowel Syndrome. Am J Gastroenterol. 2011;106:516–8. - PubMed
-
- Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–14. - PubMed
-
- Pinier M, Fuhrmann G, Galipeau HJ, et al. The copolymer P(HEMA-co-SS) binds gluten and reduces immune response in gluten-sensitized mice and human tissues. Gastroenterology. 2012;142:316–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

