The myc road to hearing restoration
- PMID: 24710525
- PMCID: PMC3901154
- DOI: 10.3390/cells1040667
The myc road to hearing restoration
Abstract
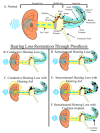
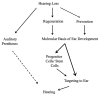
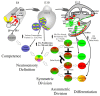
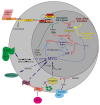
Current treatments for hearing loss, the most common neurosensory disorder, do not restore perfect hearing. Regeneration of lost organ of Corti hair cells through forced cell cycle re-entry of supporting cells or through manipulation of stem cells, both avenues towards a permanent cure, require a more complete understanding of normal inner ear development, specifically the balance of proliferation and differentiation required to form and to maintain hair cells. Direct successful alterations to the cell cycle result in cell death whereas regulation of upstream genes is insufficient to permanently alter cell cycle dynamics. The Myc gene family is uniquely situated to synergize upstream pathways into downstream cell cycle control. There are three Mycs that are embedded within the Myc/Max/Mad network to regulate proliferation. The function of the two ear expressed Mycs, N-Myc and L-Myc were unknown less than two years ago and their therapeutic potentials remain speculative. In this review, we discuss the roles the Mycs play in the body and what led us to choose them to be our candidate gene for inner ear therapies. We will summarize the recently published work describing the early and late effects of N-Myc and L-Myc on hair cell formation and maintenance. Lastly, we detail the translational significance of our findings and what future work must be performed to make the ultimate hearing aid: the regeneration of the organ of Corti.
Figures

Similar articles
-
Correct timing of proliferation and differentiation is necessary for normal inner ear development and auditory hair cell viability.Dev Dyn. 2013 Feb;242(2):132-47. doi: 10.1002/dvdy.23910. Dev Dyn. 2013. PMID: 23193000 Free PMC article.
-
N-Myc and L-Myc are essential for hair cell formation but not maintenance.Brain Res. 2012 Nov 12;1484:1-14. doi: 10.1016/j.brainres.2012.09.027. Epub 2012 Sep 25. Brain Res. 2012. PMID: 23022312 Free PMC article.
-
N-myc controls proliferation, morphogenesis, and patterning of the inner ear.J Neurosci. 2011 May 11;31(19):7178-89. doi: 10.1523/JNEUROSCI.0785-11.2011. J Neurosci. 2011. PMID: 21562282 Free PMC article.
-
Understanding the evolution and development of neurosensory transcription factors of the ear to enhance therapeutic translation.Cell Tissue Res. 2012 Aug;349(2):415-32. doi: 10.1007/s00441-012-1454-0. Epub 2012 Jun 13. Cell Tissue Res. 2012. PMID: 22688958 Free PMC article. Review.
-
Atoh1 and other related key regulators in the development of auditory sensory epithelium in the mammalian inner ear: function and interplay.Dev Biol. 2019 Feb 15;446(2):133-141. doi: 10.1016/j.ydbio.2018.12.025. Epub 2018 Dec 31. Dev Biol. 2019. PMID: 30605626 Review.
Cited by
-
Sponge bHLH Gene Expression in Xenopus laevis Disrupts Inner Ear and Lateral Line Neurosensory Development and Otic Afferent Pathfinding.Int J Mol Sci. 2025 Jun 7;26(12):5487. doi: 10.3390/ijms26125487. Int J Mol Sci. 2025. PMID: 40564948 Free PMC article.
-
Rescue from early-onset hearing loss in a mouse model lacking the cyclin-dependent kinase inhibitor p19Ink4d.Cell Death Dis. 2016 Mar 10;7(3):e2131. doi: 10.1038/cddis.2016.38. Cell Death Dis. 2016. PMID: 26962681 Free PMC article. No abstract available.
-
An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception.Diversity (Basel). 2021 Aug;13(8):364. doi: 10.3390/d13080364. Epub 2021 Aug 7. Diversity (Basel). 2021. PMID: 35505776 Free PMC article.
References
-
- Stevens G., Flaxman S., Brunskill E., Mascarenhas M., Mathers C.D., Finucane M. Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur. J. Public Health. 2011 - PubMed
-
- Corwin J.T., Cotanche D.A. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. - PubMed
-
- Ryals B.M., Rubel E.W. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. - PubMed
-
- OTOscope. [(accessed on 19 June 2012)]. Available online: http://www.healthcare.uiowa.edu/labs/morl/otoscope/home.html.
Grants and funding
LinkOut - more resources
Full Text Sources

